Introduction
Luidia is the sole genus of the family Luidiidae, characterized by long, slender, and flat arms, small paxilliform superomarginal plates that are not block-like, and knob-ending tube feet (Clark and Downey, Reference Clark and Downey1992). They are found on the sandy bottoms of the littoral to sublittoral zones and are typically distributed in the range of tropical and temperate oceans in the world (Blake, Reference Blake1983; Clark, Reference Clark1989; Lawrence, Reference Lawrence2013). The genus encompasses 48 species (Clark, Reference Clark1989; Liao and Clark, Reference Liao and Clark1995; Liu et al., Reference Liu, Liao and Li2006a, Reference Liu, Liao and Li2006b; Hopkins and Knott, Reference Hopkins, Knott, Harris, Boetger and Lesser2010; Mah and Blake, Reference Mah and Blake2012), among which, six species, viz Luidia avicularia Fisher, Reference Fisher1913, Luidia hardwicki (Gray, Reference Gray1840), Luidia maculata Müller and Troschel, Reference Müller and Troschel1842, Luidia quinaria von Martens, Reference von Martens1865, Luidia sagamina Döderlein, Reference Döderlein1920, and Luidia savignyi (Audouin, Reference Audouin1826) have been found in Japan (Kogure, Reference Kogure2018).
The genus Luidia has been divided into ten subgenera that were classified into four morphogroups; Alternata-group, Clathrata-group, Ciliaris-group, and Quinaria-group by Döderlein (Reference Döderlein1920). These subgenera were rarely referred to, and are currently considered invalid (Clark, Reference Clark1989). However, several authors have used the morphogroups for sorting of this genus (e.g., Clark, Reference Clark1953; Clark and Rowe, Reference Clark and Rowe1971; Blake, Reference Blake1973, Reference Blake1982; Xiao et al., Reference Xiao, Liu, Yuan and Sha2013), since they are morphologically distinct (Döderlein, Reference Döderlein1920). These morphogroups, however, have not been taxonomically considered as subgenera or separate genera. Recently, Xiao et al. (Reference Xiao, Liu, Yuan and Sha2013) and Lee and Shin (Reference Lee and Shin2018) used partial sequences of the mitochondrial cytochrome c oxidase subunit I gene (COI) for shedding light on the interspecific phylogeny of the Luidia species. While confirming the distinction between the Alternata- and Quinaria-groups, these analyses, however, could not completely elaborate on the phylogenetic relationships among the four morphogroups due to the low statistical supports.
In this study, we have introduced a new species of the genus Luidia found in the sublittoral waters in northern Japan. Furthermore, we revisited the classification of these morphogroups. We conducted a molecular phylogenetic analysis of the genus Luidia to verify the distinction between four morphogroups and discussed phylogenetic relationships among these groups.
Materials and Methods
Sample collection and morphological observations
The holotype of the new species was procured by the R/V Iwaki-Maru of Fukushima Prefectural Fisheries and Marine Science Research Centre from a depth of 175 m, during a bottom-trawl survey conducted on 8th March 2021. The specimen was fixed with 70% ethanol and preserved in 99.5% ethanol following procurement. The holotype specimen of the new species was deposited in the National Museum of Nature and Science, Tsukuba (NSMT).
We examined five Luidia species: including two specimens of Luidia avicularia (NSMT E-14357, 14358), one specimen of L. hardwicki (NSMT E-14359), one specimen of L. maculata (NSMT E-14356), two specimens of L. quinaria (NSMT E-14360, 14361), and one specimen of L. sagamina sagamina (NSMT E-14363) for morphological and molecular comparisons with the new species. Detailed information on the collecting location, coordinates, and dates of these specimens has been depicted in Table 1. An MZ8 dissecting microscope (Leica, Germany) was used for inspecting the specimens. Lengths of major radius (R) and minor radius (r) were measured from the centre of the mouth opening to unbroken arm tips and the connection of each proximal part of 2 arms, respectively. The arrangement of the underlying abactinal, marginal, and actinal plates were observed after removing the epidermis and paxillar spines from the proximal portions of the arms by applying commercial bleach (about 5% sodium hypochlorite). The removed spines and pedicellariae were collected in the holotype specimen of this new species for observation under a scanning electron microscope (SEM). Also, some abactinal, superomarginal, and inferomarginal plates were isolated to observe their sizes and shapes. These isolated spines, pedicellariae, and plates were immersed in a drop of commercial bleach for a few minutes which was followed by washing with deionized water to clean remnant tissues. The samples were then mounted on brass SEM stubs and desiccated in the air. Finally, these ossicles were observed under a JSM-6380LV SEM (JEOL, Japan) after coating them with gold-palladium. The semi-dried holotype specimen was scanned using an inspeXio SMX-225CT FPD HR micro-computed tomography (micro-CT) scanner (Shimadzu, Japan), at a tube voltage of 115 kV and a tube current of 70 μA for 30 min, which revealed the fasciolar grooves between the inferomarginal plates. Three-dimensional images were reconstructed with the software VGSTUDIO Max 3.2 (Volume Graphics, Germany).
Table 1. Specimens examined for morphological comparisons with Luidia iwakiensis n. sp

DNA extraction, gene amplification, and molecular analysis
DNA was extracted from chopped tube feet of six Japanese Luidia species (NSMT E-14356, NSMT E-14357, NSMT E-14359, NSMT E-14360, NSMT E-14363, and NSMT E-14364) by boiling the tissues with Chelex-100 resin (Bio-Rad, Inc., USA) in ultrapure water for 30 min, followed by rapid cool down on the ice for ten minutes. Polymerase chain reaction (PCR) was carried out by using 5.0 μl of 2 × PCR Buffer (Takara Bio, Inc., Japan), 0.2 μl TKs Gflex Polymerase (Takara Bio, Inc., Japan), 0.2 μl of each primer pair (10 μM), 1.0 μl of extracted DNA, and 3.4 μl of ultrapure water (3.4 μl). Two primer pairs, COIceF (5'-ACTGCCCACGCCCTAGTAATGATATTTTTTATGGTNATGCC-3') and COIceR (5'-TCGTGTGTCTACGTCCATTCCTACTGTRAACATRTG-3'), and 16SaL (5'- CGCCTGTTTATCAAAAACAT −3') and 16SAN-R (5'- GCTTACGCCGGTCTGAACTCAG -3') targeted the partial sequences of the mitochondrial COI and 16S rRNA gene (16S), respectively (Palumbi, Reference Palumbi1994; Zanol et al., Reference Zanol, Halanych, Struck and Fauchald2010; Hoareau and Boisson, Reference Hoareau and Boissin2010). The protocol used for PCR amplification was as follows: preheating at 94 °C for 1 min; 30 cycles of 98 °C for 10 s, 55 °C for 15 s, and 68 °C for 30 s. Following purification using ExoSAP-IT Cleanup Regent (Thermo Fisher Scientific, Inc., USA), Big Dye Terminator v3.1 Cycle Sequencing Kit (Thermo Fisher Scientific, Inc., USA) was used for sequencing the PCR products using the Applied Biosystems 3500xL Genetic Analyzer (Life Technologies, Inc., USA). Assembly of the sequenced data was carried out using GeneStudio Professional Edition ver. 2.2.0.0 (GeneStudio, Inc., USA), which successfully provided COI (598–709 bp) and 16S (555–638 bp) sequences of all specimens. In addition, 15 registered sequences from the GenBank were obtained, and a total of 21 sequences for each gene marker were aligned by MAFFT ver. 7.222 (Katoh and Standley, Reference Katoh and Standley2013). The outgroups in our datasets were represented by two species with registered COI and 16S sequences, that belonged to the closest clades of the paraphyletic sister family Astropectinidae to the family Luidiidae (Mah and Foltz, Reference Mah and Foltz2011). For the COI dataset, the stop codons were removed by manually editing the sequence errors using MEGA ver. 7.0.26 (Kumar et al., Reference Kumar, Stecher and Tamura2016). The 16S dataset was trimmed with the gappyout option by using trimAL 1.2rev59 (Capella-Gutiérrez et al., Reference Capella-Gutiérrez, Silla-Martínez and Gabaldón2009). GTR + G was selected as a best-fit substitution model by Kakusan4 (Tanabe, Reference Tanabe2011) to construct phylogeny for the maximum likelihood (ML) analysis. Both datasets were partitioned by gene region, and the COI dataset was additionally partitioned based on the codon position. ML tree was inferred using RAxML ver. 8.2.9 (Stamatakis, Reference Stamatakis2014). Bootstrap values (BS) were calculated from 1000 replicates. MEGA ver. 7.0.26 (Kumar et al., Reference Kumar, Stecher and Tamura2016) was used to calculate the interspecific Kimura 2-parameter (K2P) distance.
Results
Systematics
Family Luidiidae Forbes, Reference Forbes1839
Genus Luidia Forbes, Reference Forbes1839
Luidia iwakiensis n. sp.
(https://zoobank.org/NomenclaturalActs/9B98B65F-6D0E-4666-938FC21890F172BA)
(New Japanese name: Sazare-suna-hitode)
Type Material
Holotype. NSMT E-14364, off Iwaki, Fukushima Prefecture, Japan, 36°53.41’N, 141°16.44’E (Figure 1), 175 m, on March 8, 2021, 3 arms were detached, fixed in 99.5% ethanol, and 1 detached arm was dried.

Figure 1. Locality of sampling site (indicated by solid stars) of Luidia iwakiensis n. sp.
Etymology
The specific name derives from the type locality, Iwaki City in Fukushima Prefecture, Japan. The Japanese name ‘sazare’ is derived from the pebbles called ‘sazare-ishi’ in Japanese, referring to the numerous pebble-like pedicellariae on the abactinal side. ‘Suna-hitode’ comes from the Japanese name of the genus Luidia.
Diagnosis
Arms five, slender. Abactinal spines almost uniform in size and shape. Abradial-most abactinal series contains 1.5 times or more plates than adjacent superomarginal series. Major inferomarginal spines longest in abactinal-most one, as long as nearby 2–3 inferomarginal plates. Minor inferomarginal spines half lengths of inferomarginal fasciolar grooves having equal depth and length. Pedicellariae present on proximal to middle abactinal, almost all actinal, and all oral plates. Body colour solid, lacking pattern.
Description
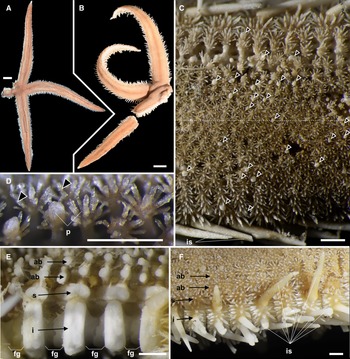
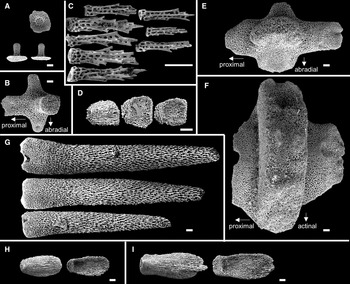
Arms five, flat, slender, and gradually tapering to the arm tip (Figures 2A, B & 3A). R 86.3 mm, r 9.3 mm, and R/r ratio 9.3r. Abactinal surface covered by numerous paxillae (Figure 2C, 2D). These paxillae composed of abactinal plates bearing spines and no or one pedicellaria on pawn-shaped ridges (Figure 4A, 4B). Abactinal plates round or quadrilobate. Round abactinal plates bearing 9–17 abactinal spines irregularly arranged in disc and along midradial line of arms. Quadrilobate plates bearing 15–23 abactinal spines arranged in 2–3 longitudinal series in abactinolateral portion of arm. Quadrilobate abactinal plates three times longer and wider than round abactinal plates. Abactinal spines 0.2–0.3 mm in length, straight, cylindrical, and uniformly smooth, except for distal half of spines with sparse serration by splayed thorns (Figure 4C). Abactinal pedicellariae round-shaped and bivalvate or trivalvate (Figure 4D). Each papular area contains no or one branched papula. Madreporite located at the extremity of the disc.

Figure 2. Luidia iwakiensis n. sp., holotype, NSMT E-14364. A, Whole body of live specimen, abactinal view, B, whole body of ethanol preserved specimen, abactinal view; C, abactinal surface of the middle portion of the arm, showing abactinal pedicellariae (arrowheads); D, abactinal paxillae from the proximal portion of arm, showing abactinal pedicellariae (arrowheads); E, denuded abactinal to lateral surface of the middle portion of arm; F, lateral to abactinal surface of the proximal portion of arm. Abbreviations: ab, abactinal series; fg, fasciolar groove; i, inferomarginal series; is, major inferomarginal spines; p, paxilla; s, superomarginal series. Scale bars indicate 10 mm for A, B, 1 mm for C–F. Proximal is left in C–F.

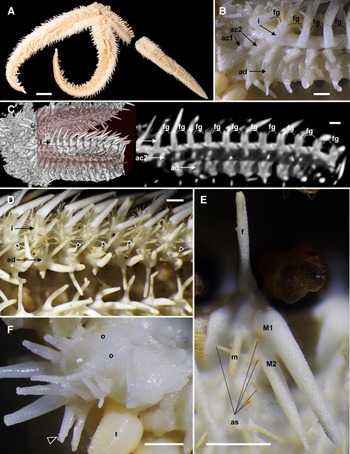
Figure 3. Luidia iwakiensis n. sp., holotype, NSMT E-14364. A, Whole body of ethanol preserved specimen, actinal view; B, denuded actinal surface of the proximal portion of the arm; C, horizontal section of the denuded inferomarginal plates using micro-CT (right), showing the sectioned plane (left, red rectangle); D, actinal surface of the proximal portion of arm, showing actinal pedicellariae (arrowheads); E, spines on adambulacral and actinal plates; F, oral region, showing oral pedicellariae (arrowheads). Abbreviations: ac1, first actinal series; ac2, second actinal series; ad, adambulacral series; as, actinal spines; f, furrow spine; fg, fasciolar groove; i, inferomarginal series; M1, major subambulacral spine positioned adradially; M2, major subambulacral spine positioned abradially; m, minor subambulacral spines; o, oral plates; t, tube feet. Scale bars indicate 10 mm for A, 1 mm for B–F. Proximal is left in B–F.

Figure 4. SEM images of ossicles of Luidia iwakiensis n. sp., holotype, NSMT E-14364. A, Round abactinal plates, abactinal (above) and lateral (below two) views; B, quadrilobate abactinal plate in the abradial-most abactinal series; C, abactinal spines; D, detached valves of the abactinal pedicellaria, outside (left one) and inside (right two) views; E, superomarginal plate, abactinal view; F, inferomarginal plate, lateral view; G, major inferomarginal spines on the inferomarginal plate shown in F, borne on the abactinal (above), the lateral (middle), and the actinal (below) area of the plate; H, detached vaves of the actinal pedicellaria, outside (left) and inside (right) views; I, detached valves of the oral pedicellaria, outside (left) and inside (right) views. Ossicles in A–B and E–G were collected from the middle portion of arm showed in Figure 2D. Scale bar indicates 0.1 mm in all images. Bottom is left in C, D, G–I.
Superomarginal plates longitudinally elongated, two times longer than the adjacent abactinal plates, quadrilobate with crescent-shaped rides (Figure 4E), and arranged in one longitudinal series at abactinolateral portion of arm (Figure 2E, 2F). Each superomarginal series composed of 68–74 plates, corresponding to 140–149 abactinal plates of abradial-most abactinal series. Inferomarginal plates transversely elongated, two times wider than adjacent superomarginal pates, quadrilobate with oblong ridges (Figure 4F), and arranged in one longitudinal series at lateral portion of arms (Figures 2E & 3B). U-shaped fasciolar grooves between ridges of two consecutive inferomarginal plates have equal depth and length (Figures 2E & 3B, 3C). Each superomarginal plate possesses 21–38 spines. Each inferomarginal plate bears three major spines and numerous minor spines (Figures 2F & 3D). Major inferomarginal spines 1.5–3.6 mm in length, straight, conical, and uniformly smooth (Figure 4G). These spines arranged in one vertical row on each inferomarginal plate, and these rows alternately positioned abactinally and actinally on lateral sides of neighbouring inferomarginal plates. Abactinal-most major inferomarginal spines longest, as long as 2–3 nearby inferomarginal plates (Figures 2F & 3F, 3G). Minor spines closely located on surfaces of inferomarginal ridges and densely filled fasciolar grooves (Figure 2F). Minor spines half lengths of fasciolar grooves. Pedicellariae absent on all supero- and inferomarginal plates.
Actinal plates transversely elongated, ovoid with round ridges, and arranged in two longitudinal series (Figure 3B). First series composed of only two plates confined within each interradial disc. Second series exceeds two-thirds of arm length, reaching nearly to arm tips. Each actinal plate bears three to six spines (Figure 3E), and most actinal plates bear one bicuspid pedicellaria (Figures 3D & 4H).
Adambulacral plates transversely elongated, longitudinally constricted at median part of each plate, and arranged in one longitudinal series along ambulacral furrow (Figure 3B, 3C). Adambulacral plates constantly bear one furrow and two major subambulacral spines arranged in one transverse row and one or two minor subambulacral spines positioned proximal to this row (Figure 3D, 3E). These spines conical and uniformly smooth as major inferomarginal spines, however, furrow and first subambulacral spines curved. Pedicellariae absent on all adambulacral plates.
Each oral plate bears 10–18 oral spines arranged in two rows (Figure 3F). Each oral spine straight, conical, uniformly smooth, and gradually decreases in size towards distal side of each plate. One bicuspid pedicellaria present on the proximal edge of each oral plate, one-third of length of oral plates (Figures 3F & 4I).
Colour in living and ethanol-preserved specimen appears uniform yellow with dark midradial line on abactinal side and white on actinal side (Figures 2A, 2B & 3A). Abactinal-most inferomarginal spines yellow and become gradually white coloured towards tips (Figure 2F).
Distribution
Luidia iwakiensis n. sp. has only been known from the type locality, off Iwaki, Fukushima Prefecture, Japan, at a depth of 175 m.
Phylogenetic analysis
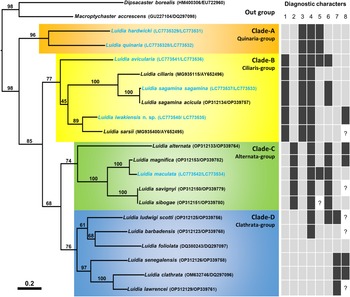
The phylogenetic tree (Figure 5) demonstrated four distinct clades (A–D) with moderate to high bootstrap supporting values (74–96%). Of these four clades, Clade-A was branched from the others at first and Clade-B was branched at second. A sister relationship between Clade-C and Clade-D was supported with moderate bootstrap values (68%).

Figure 5. Molecular phylogenetic tree of 18 Luidia species based on concatenated sequences of COI and 16S genes (1207 bp) by the maximum likelihood analysis. The values on branches indicate bootstrap support. The scale bar indicates substitutions per site in branch length. GenBank accession numbers of COI and 16S are depicted before and after the slashes, respectively, in parentheses. Blue OTUs indicate the sequences newly obtained in this study. Presence (black rectangle), absence (grey rectangle), or both (black and grey) of eight diagnostic characters of morphogroups indicated by Döderlein (Reference Döderlein1920) are shown for each species: 1, the abradial-most abactinal series contains 1.5 times or more plates than the adjacent superomarginal series; 2, mottled and/or banded colour pattern; 3, oral pedicellariae; 4, actinal pedicellariae; 5, adambulacral pedicellariae; 6, enlarged abactinal spines on the centre of paxillae; 7, two furrow spines on each adambulacral plate; 8, two or more actinal series (Perrier, Reference Perrier1884; Koehler, Reference Koehler1895; Fisher, Reference Fisher1906b, Reference Fisher1911, Reference Fisher1913, Reference Fisher1919; Goto, Reference Goto1914; Döderlein, Reference Döderlein1920; Mortensen, Reference Mortensen1933; Clark, Reference Clark1953, Reference Clark1982; Clark and Rowe, Reference Clark and Rowe1971; Downey, Reference Downey1973; Hayashi, Reference Hayashi1973; Walenkamp, Reference Walenkamp1976; Clark and Downey, Reference Clark and Downey1992; Liao and Clark, Reference Liao and Clark1995; Hopkins and Knott, Reference Hopkins, Knott, Harris, Boetger and Lesser2010; Kogure, Reference Kogure2015). Question marks mean unknown.
Among the eighteen analysed species, L. hardwicki and L. quinaria, belonged to Clade-A, L. avicularia, L. ciliaris (Philippi, Reference Philippi1837), L. iwakiensis n. sp., L. sagamina, and L. sarsii Düben & Koren in Düben, Reference Düben1844, belonged to Clade-B, L. alternata (Say, Reference Say1825), L. maculata, L. magnifica Fisher, Reference Fisher1906b, L. savignyi, and L. sibogae Döderlein, Reference Döderlein1920, belonged to Clade-C, and L. barbadensis Perrier, Reference Perrier1881, L. clathrata (Say, Reference Say1825), L. foliolata Grube, Reference Grube1866, L. lawrencei Hopkins and Knott, Reference Hopkins, Knott, Harris, Boetger and Lesser2010, L. ludwigi Fisher, Reference Fisher1906a, and L. senegalensis (de Lamarck, Reference de Lamarck1816), belonged to Clade-D (Figure 5). The new species, L. iwakiensis n. sp., was most closely related to L. sarsii with 13.8% in K2P distance.
Discussion
Molecular and morphological evidence for 4 species groups within Luidia
Four monophyletic clades were evident from our molecular analysis (Figure 5). A morphological comparison of species belonging to these four clades by observing eight specimens of six Japanese species (Table 1) and surveying previous descriptions of each species (see the legend of Figure 5) exhibited three characters among the eight characters outlined in Döderlein's (Reference Döderlein1920) diagnosis clearly defined each clade. These three characteristics were as follows: presence/absence of elongated superomarginal plates corresponding to 1.5 times or more abactinal plates, a mottled and/or banded pattern on ethanol specimens, and oral pedicellariae (Figure 5). Based on these three diagnostic characters, Clade-A, B, C, and D were identified with the Quinaria-, Ciliaris-, Alternata-, and Clathrata-groups, respectively, and Luidia species were re-classified into these morphogroups as shown in Table 2. However, the remaining five characters indicated by Döderlein (Reference Döderlein1920); which included the presence/absence of enlarged spines on the paxillae, presence of pedicellariae on the actinal and adambulacral plates, presence of two furrow spines, and two or more actinal series were inconsistent with the clades supported by molecular data.
Table 2. An updated classification of Luidia species into four morphogroups based on three diagnostic characters (1, the abradial-most abactinal series contains 1.5 times or more plates than the adjacent superomarginal series; 2, mottled and/or banded colour pattern; 3, oral pedicellariae).
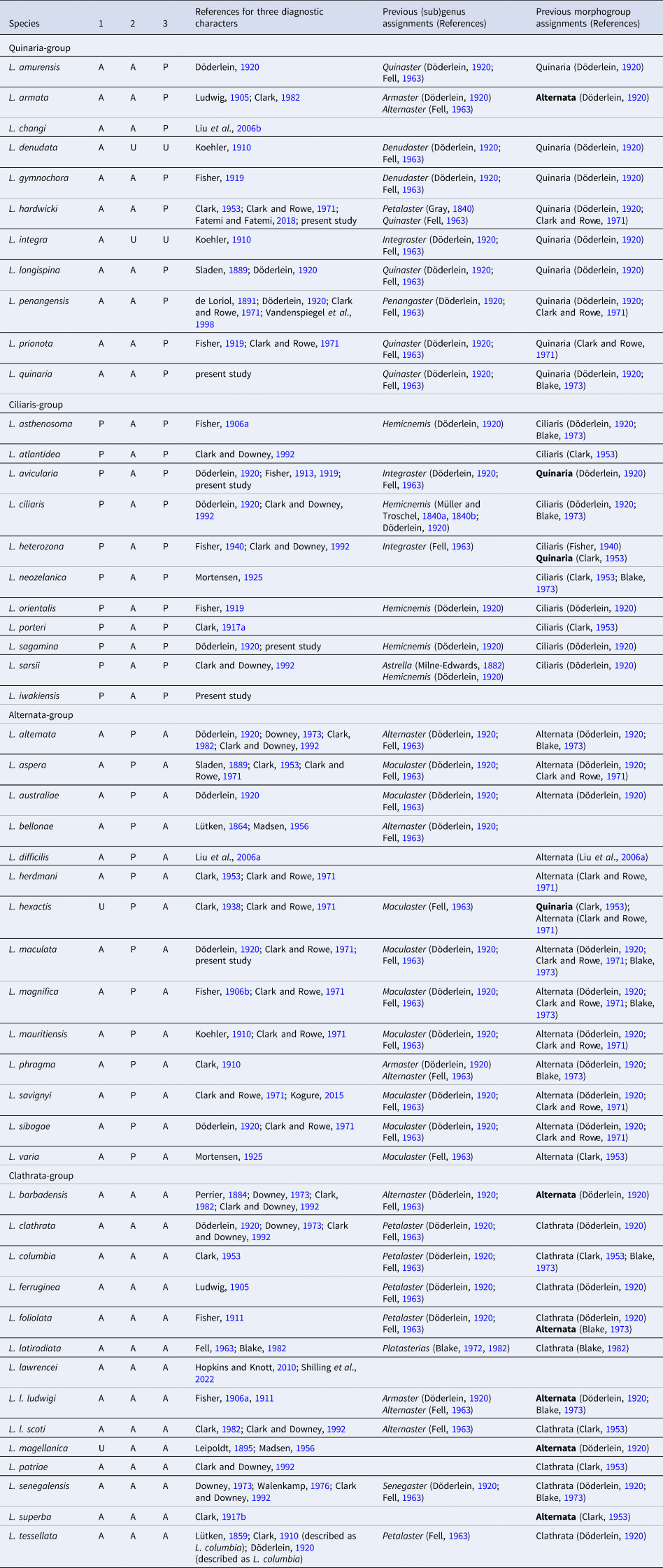
Following Blake's (Reference Blake1972, Reference Blake1982) classification, we include Luidia latiradiata here, while this species has been classified as Somasteroidea (Fell, Reference Fell1963). P: presence, A: absence, U: unknown. Previous assignments to genus or subgenus (currently synonymized with Luidia) and morphogroup (if different in bold) are also shown.
In our phylogenetic tree, the Quinaria-group was at the most basal position, and the clades of the the Alternata- and Clathrata-group were at the terminal (Figure 5). Alternata- and Clathrata-groups were also placed at the terminal position in a report by Lee and Shin (Reference Lee and Shin2018). In accordance with the original suggestions of Döderlein (Reference Döderlein1920), the Clathrata-group was basal since it shared the absence of pedicellariae with the other Paxillosida species. Xiao et al. (Reference Xiao, Liu, Yuan and Sha2013) placed the representative species of the Alternata- and Clathrata-groups in the basal position, and Blake (Reference Blake1973) indicated some ossicle similarities between Alternata- and Clathrata-groups and the other Paxillosida species, thereby supporting this hypothesis. In contrast with this hypothesis, our molecular tree indicated two groups with oral pedicellariae, Quinaria- and Ciliaris-groups, were basal in the genus Luidia. This suggested that oral pedicellariae might have evolved in the common ancestor of Luidia, and secondarily lost in the Alternata- and Clathrata-groups.
The distinct characters of the four morphogroups of Luidia, as proposed by Döderlein (Reference Döderlein1920), find strong support from our molecular analysis, suggesting that these morphogroups could be classified as separate taxa. Twelve synonymized genera or subgenera were established in the genus Luidia by previous papers, and we assigned them into four morphogroups after reviewing the descriptions of type species as follows:
(1) Quinaria-group: Petalaster Gray, Reference Gray1840, Armaster Döderlein, Reference Döderlein1920, Denudaster Döderlein, Reference Döderlein1920, Integuraster Döderlein, Reference Döderlein1920, Penangaster Döderlein, Reference Döderlein1920, Quinaster Döderlein, Reference Döderlein1920.
(2) Ciliaris-group: Luidia Forbes, Reference Forbes1839, Astrella Perrier, Reference Perrier1882, Hemicnemis Müller & Troschel, 1840.
(3) Alternata-group: Alternaster Döderlein, Reference Döderlein1920, Maculaster Döderlein, Reference Döderlein1920.
(4) Clathrata-group: Platasterias Gray, Reference Gray1871, Senegaster Döderlein, Reference Döderlein1920
However, since we analysed specimens of only a limited number of Luidia species, we hesitate to classify the morphogroups as separate subgenera or genera herein.
Comparisons of new species with the other species
Luidia iwakiensis n. sp. is placed within the Ciliaris-group in having the abradial-most abactinal series composed of 1.5 times or more numerous plates than the adjacent superomarginal series, the pedicellariae on the oral plates, and the solid body colour lacking pattern (Figures 2A, 2B, 2D & 3F). Fourteen species and subspecies of Luidia were assigned to the Ciliaris-group: eight Atlantic species and subspecies, Luidia atlantidea Madsen, 1950; Luidia ciliaris (Philippi, Reference Philippi1837); Luidia heterozona barimae John and Clark, Reference John and Clark1954; Luidia heterozona heterozona Fisher, Reference Fisher1940; Luidia sagamina acicula Mortensen, Reference Mortensen1933; Luidia sarsii sarsii Düben & Koren in Düben, Reference Düben1844; Luidia sarsii africana Sladen, Reference Sladen1889; Luidia sarsii elegans Perrier, 1875; six Pacific species, Luidia asthenosoma Fisher, Reference Fisher1906a; Luidia avicularia Fisher, Reference Fisher1913; Luidia neozelanica Mortensen, Reference Mortensen1925; Luidia orientalis Fisher, Reference Fisher1913; Luidia porteri Clark, Reference Clark1917a; Luidia sagamina sagamina Döderlein, Reference Döderlein1920 (Table 2). Among these species, L. avicularia, L. ciliaris, L. h. barimae, and L. h. heterozona have six to ten arms, and can be readily distinguished from the L. iwakiensis n. sp. (Fisher, Reference Fisher1913, Reference Fisher1919, Reference Fisher1940; Döderlein, Reference Döderlein1920; John and Clark, Reference John and Clark1954; Clark and Downey, Reference Clark and Downey1992). Furthermore, L. atlantidea, L. neozelanica, L. orientalis, L. s. acicula, and L. s. sagamina are clearly discriminated from L. iwakiensis n. sp. by having the abactinal-most major inferomarginal spines longer than three nearby inferomarginal plates (Fisher, Reference Fisher1919; Clark, Reference Clark1917a; Döderlein, Reference Döderlein1920; Mortensen, Reference Mortensen1925; Clark and Downey, Reference Clark and Downey1992; Gallardo-Roldán et al., Reference Gallardo-Roldán, García, Lozano, Antit, Baro and Rueda2015). The presence/absence of pedicellariae enables the distinction of the new species from the remaining five related species and subspecies.
Luidia iwakiensis n. sp. has pedicellariae on proximal to middle abactinal, almost all actinal, and all oral plates, whereas L. asthenosoma and L. porteri also have pedicellariae on the supero- and inferomarginal plates (Fisher, Reference Fisher1906a; Clark, Reference Clark1917a), and L. s. africana, L. s. elegans, and L. s. sarsii lack pedicellariae on the middle to distal actinal plates (Clark, Reference Clark1982). Clark (Reference Clark1953) pointed out that the presence of pedicellariae varies based on the body size in some Luidia species. Comparison of similar-sized specimens of the holotypes of L. iwakiensis n. sp. (R = 86.3 mm), L. asthenosoma (R = 86 mm in Fisher, Reference Fisher1906a), L. porteri (R = 98 mm in Clark, Reference Clark1917a), and three subspecies of L. sarsii (R = 90–190 mm in Clark and Downey, Reference Clark and Downey1992) confirmed the above-mentioned differences with regards to the occurrence of pedicellariae.
Our phylogenetic analysis based on COI and 16S gene markers supported the morphological classification of Luidia iwakiensis n. sp. as a member of the Ciliaris-group and demonstrated relationship between L. iwakiensis n. sp. and the Atlantic species L. sarsii (Figure 5). The K2P genetic distance between L. iwakiensis n. sp. and L. sarsii was 13.8%, which was long enough to consider them as genetically and geographically distinct species (Hebert et al., Reference Hebert, Cywinska, Ball and deWaard2003; Shilling et al., Reference Shilling, Krueger-Hadfield and McClintock2022).
Key to the recognized species of Luidia in Japanese waters
In this study, we report a seventh Luidia species in Japanese waters. This key to the seven Japanese species is based on our observations and previous descriptive papers (Döderlein, Reference Döderlein1920; Liao & Clark, Reference Liao and Clark1995; Hayashi, Reference Hayashi1973; Kogure, Reference Kogure2015).
1. Superomarginal plates that are shorter than or as long as the adjacent abactinal plates; the abradial-most abactinal series and superomarginal series contain almost identical numbers of plates 2
– Superomarginal plates are considerably longer than the adjacent abactinal plates; the abradial-most abactinal series contains 1.5 times or more plates than the adjacent superomarginal series; oral plates bear pedicellariae (Ciliaris-group) 5
2. Oral plates bear pedicellariae; abactinal surface lack mottled and/or banded colour pattern (Quinaria-group) 3
– Oral plates lack pedicellariae; abactinal surface has mottled and/or banded colour pattern (Alternata-group) 4
3. Adambulacral plates lack pedicellariae or only proximal 1–3 adambulacral plates bear pedicellariae; furrow and subambulacral spines are arranged in 1 or 2 zigzag transverse rows; abactinal surface is uniform orange-cinnamon L. hardwicki
– Adambulacral plates bear pedicellariae; furrow and subambulacral spines are arranged in a transverse row; abactinal surface is brown with a longitudinal dark stripe along the midradial line L. quinaria
4. Arms 6; some abactinal paxillae bear enlarged central spines L. savignyi
– Arms 7–9; abactinal paxillae lack enlarged central spines L. maculata
5. Arms 9–10 L. avicularia
– Arms 5 6
6. Abactinal plates lack pedicellariae; the abactinal-most major inferomarginal spines longer than three nearby inferomarginal plates L. s. sagamina
– Abactinal plates bear pedicellariae; the abactinal-most major inferomarginal spines as long as 2–3 nearby inferomarginal plates L. iwakiensis n. sp.
Data availability
The data that support the findings of this study are available from the corresponding author, IK, upon reasonable request.
Acknowledgements
We would like to express our sincere gratitude to Takashi Iwasaki, Masato Ikegawa, and the captain and crew of R/V Iwaki-Maru (Fukushima Prefectural Fisheries and Marine Science Research Centre) for providing the holotype of Luidia iwakiensis n. sp. (NSMT E-14364). We also thank to the captain and crew of following research vessels for collecting specimens: R/V Seisui-Maru (Mie University): L. avicularia (NSMT E-14357, 14358) and L. quinaria (NSMT E-14361); R/V Toyoshio-Maru (Hiroshima University): L. sagamina sagamina (NSMT E-14363) and L. quinaria (NSMT E-14360); R/V Shinsei-Maru (Japan Agency for Marine-Earth Science and Technology): L. hardwicki (NSMT E-14359); R/V Koyo (Tokyo Metropolitan Ogasawara Fisheries Center): L. maculata (NSMT E-14356). We acknowledged the following researchers for managing research cruise and/or providing processed specimens: NSMT: Akito Ogawa, Hironori Komatsu, and Mikihito Arai; Hiroshima University: Susumu Otsuka; Mie University: Shoichi Kimura and Taeko Kimura. We are grateful to Gento Shinohara, Shuhei Nomura, Takahiko Kutsuna, and Yasunari Shigeta (NSMT) for their help in manipulating micro-CT, and Takeshi Furukawa, Masamitsu Iwata, Makoto Kuraishi, and Subaru Joukura (Marine Science Museum, Fukushima Prefecture, Aquamarine Fukushima) for their helpful advice and encouragement. We appreciate the suggestive and helpful comments on the present study by anonymous reviewers. The authors would like to thank Enago (www.enago.jp) for the English language review.
Authors’ contributions
MH provided the specimen of L. iwakiensis n. sp. All authors performed morphological observations and molecular analyses. IK and TF drafted this manuscript. All authors commented on and approved the final manuscript.
Financial support
This study was partly supported by JSPS KAKENHI Grant Number 21K06327 and the National Museum of Nature and Science projects ‘Geological, biological, and anthropological histories in relation to the Kuroshio Current’, ‘Biological Properties of Biodiversity Hotspots in Japan’, and ‘Collection of mitochondrial genome data of invertebrates and its application to taxonomy and phylogeny’.
Competing interests
None.
Ethical standards
Not applicable.