Introduction
Distolambrus maltzami (Miers, Reference Miers1881) (Family Parthenopidae) is a small (carapace width of <15 mm), highly distinctive brachyuran crab found in the Eastern Atlantic Ocean and the Mediterranean Sea (d'Udekem d'Acoz, Reference d'Udekem d'Acoz1999; Pipitone & Arculeo, Reference Pipitone and Arculeo2003; Massi et al., Reference Massi, Micalizzi, Giusto and Pipitone2010). This species was originally described from specimens collected off the west coast of Africa and named after Baron Hermann-Maltzan, although the specific name has appeared in subsequent literature with alternative spellings (‘maltzani’ and ‘maltzami’) until it was resolved in Manning & Holthuis (Reference Manning and Holthuis1981) as Heterocrypta maltzami. A new genus (Distolambrus) was erected for this species by Tan & Ng (Reference Tan and Ng2007) based on a unique combination of morphological characters including the distinct carapace shape.
Distolambrus maltzami has a very wide geographic distribution in the Eastern Atlantic Ocean and Mediterranean. Originally described from Gorée Island, Senegal from depths of 18–28 m (Miers, Reference Miers1881), the species has been found along the western coast of Sub-Saharan Africa, extending from the Republic of Congo (Balss, Reference Balss1921) to Mauritania (Manning & Holthuis, Reference Manning and Holthuis1981; De Matos-Pita et al., Reference De Matos-Pita, Castillo and Ramil2017); and around the Cape Verde (Milne-Edwards & Bouvier, Reference Milne-Edwards and Bouvier1894) and Azores archipelagos (Ortmann, Reference Ortmann1894). In the same year as Miers' discovery off Western Africa, another putative Heterocrypta species, Heterocrypta marioni (Milne-Edwards, Reference Milne-Edwards1881, Reference Milne-Edwards1882) was recorded in the Mediterranean, south of Toulon, France, at a depth of 455 m (Milne-Edwards, Reference Milne-Edwards1881). Milne-Edwards & Bouvier (Reference Milne-Edwards and Bouvier1900) subsequently synonymized Heterocrypta marioni with Heterocrypta maltzami, although some authors (e.g. d'Udekem d'Acoz, Reference d'Udekem d'Acoz1999) continued to recognize northern specimens as a variety of H. maltzami, under the name ‘Heterocrypta maltzami var. marioni’. The name Heterocrypta marioni is currently recognized as a synonym of Distolambrus maltzami (WoRMS, 2023).
The overall bathymetric range of D. maltzami is very broad. Most of the tropical and subtropical records from West Africa have tended to be from relatively shallow waters, from the sublittoral to 100 m (Manning & Holthuis, Reference Manning and Holthuis1981), but sometimes as deep as 347 m, off the Cape Verde Islands (Milne-Edwards & Bouvier, Reference Milne-Edwards and Bouvier1900). Recent records give a more extensive depth range of 22–550 m (De Matos-Pita et al., Reference De Matos-Pita, Castillo and Ramil2017) with a trend for relatively shallow <100 m depth records (Massi et al., Reference Massi, Micalizzi, Giusto and Pipitone2010; Santin et al., Reference Santin, Aguilar, Akyol, Begburs, Benoit, Chimienti, Crocetta, Dalyan, De La Linde Rubio, Dragičević, Dulčić, Giglio, Gönülal, Kebapcioglu, Kesici, Kiparissis, Kousteni, Mancini, Mastrototaro, Menut, Montesanto, Peristeraki, Poursanidis, Renoult, Sánchez-Tocino, Sperone and Tiralongo2021) but also includes specimens from as deep as 412 m (Massutí et al., Reference Massutí, Sánchez-Guillamón, Farriols, Palomino, Frank, Bárcenas, Rincón, Martínez-Carreño, Keller, López-Rodríguez and Díaz2021). This crab has been reported from a range of seabed types, including shell debris, shelly sand, sand, muddy sand, mud, calcareous algae, coral and rock (Pesta, Reference Pesta1918; Manning & Holthuis, Reference Manning and Holthuis1981).
Previously, Distolambrus maltzami had been collected in the Bay of Biscay but very infrequently, with only three records in 1886 and 1887 (Milne-Edwards & Bouvier, Reference Milne-Edwards and Bouvier1894), one in 1964 (Lagardere, Reference Lagardere1973) and another in 1994 (Paulmier, Reference Paulmier1997), although this apparent lack of records could be an artefact of sampling with larger mesh trawling gear. This paper provides an update to the known distribution of D. maltzami with many records from the Bay of Biscay and the Celtic Sea, including the first record of this species in UK seas. To complement describing the species morphology and reporting new records, we include a key to distinguish it from similar, related parthenopid species in the North-east Atlantic. Additionally, with more records and information on the environmental conditions in which they occur, we can predict where this species may be found. We modelled the distribution of D. maltzami in the Bay of Biscay and the Celtic Sea by combining (i) absence/presence records of the species from the French scientific trawl surveys ‘EValuation Halieutique de l'Ouest Européen’ (EVHOE survey; 2008–2018) and (ii) a collation of substrate and ocean features that are considered relevant to characterize benthic environmental conditions that would create a suitable habitat for D. maltzami. This combination of proven and predicted distribution will facilitate a greater understanding of the drivers of its current distribution pattern and thus highlight the potential for encountering D. maltzami during fishery monitoring surveys in the region.
Materials and methods
The Institut Français de Recherche pour l'Exploitation de la Mer (IFREMER) EVHOE survey series (Laffargue et al., Reference Laffargue, Salaun, Garren, Bellail, Mahe and Poulard1987) within the Bay of Biscay and Celtic Sea employs a Grande Ouverture Verticale (GOV) fishing net with a horizontal opening of 20 m, a vertical opening of 4 m and a cod-end mesh of 20 mm (ICES, 2017). The Centre for Environment, Fisheries and Aquaculture Science (CEFAS) Quarter One South West EcoSystem (Q1SWECOS) survey samples the Western Channel and Celtic Sea. This survey uses two commercially rigged 4 m beam trawls with 80 mm cod-end mesh, with one of the trawls fitted with a 40 mm liner to facilitate the collection of epibenthic species. Both the CEFAS and IFREMER survey series complement each other in allowing for assemblages of larger epibenthic fauna to be routinely monitored, in support of an ecosystem-approach to fisheries data collection and assessment, and provide data for an extensive area (Figure 1) comprising the continental margin of the Bay of Biscay (Golfe de Gascogne), Celtic Sea (Mer Celtique) and western part of the English Channel (La Manche) where the seabed is suitable for successful deployment of beam trawl or GOV fishing nets. The covered bathymetric range is ~20–530 and 20–200 m for the EVHOE and Q1SWECOS surveys, respectively.

Fig. 1. Trawl locations from the CEFAS Q1SWECOS 4 m beam trawl surveys (2013–2021, black circles) and IFREMER EVHOE GOV surveys (2008–2020, grey circles), overlain on bathymetry from GEBCO (Becker et al., Reference Becker, Sandwell, Smith, Braud, Binder, Depner, Fabre, Factor, Ingalls, Kim, Ladner, Marks, Nelson, Pharaoh, Trimmer, Von Rosenberg, Wallace and Weatherall2009), showing the capture locations of Distolambrus maltzami (Miers, Reference Miers1881) within the Celtic Sea and the Bay of Biscay (red circles).
Sampling of benthos only recently became an integral part of these surveys, with identification and quantification of full benthic sorts from 2008 and 2013 for the EVHOE and the Q1SWECOS surveys, respectively. These surveys encompass a vast area and provide platforms for collecting spatially-comprehensive data to evaluate the presence and distribution of species such as D. maltzami in the region (Figure 1), however, it must be recognized that species' catchability, their locality and the fishing gear deployed have a large influence on the benthos caught.
To create the species distribution model, environmental variables were selected using a stepwise process based on Variance Inflation Factor (VIF). This was to maintain the lowest multicollinearity (VIF scores below 10) among the variables, as values above 10 indicate high correlation (Craney & Surles, Reference Craney and Surles2002). The final set of environmental variables included horizontal current velocity (standard deviation, minimum, maximum and median values), chlorophyll-a concentration (minimum and maximum values), annual temperature (minimum and median values), oxygen concentration (standard deviation and median values), nitrate concentration (minimum and maximum values) and seabed substrate. All oceanic biophysical variables are derived from bottom layer ocean model outputs and correspond to climatological summary statistics (EU Copernicus Marine Services Information, 2021) derived from monthly outputs between 2008 and 2018 for a resolution of 0.083 × 0.083° (~10 km) (downloaded from: https://resources.marine.copernicus.eu/products). Seabed substrate information was derived from the EMODnet Geology ‘Seabed substrate’ product, in which substrate types are described according to a Folk 7-class classification (downloaded from: https://www.emodnet-geology.eu/data-products/seabed-substrates/).
The modelling was performed by temporally aggregating presence/absence data of the species from the EVHOE scientific campaign records for the period 2008–2018. This created 67 presence and 107 absence records gridded within 6.4 × 6.4 km quadrat cells to thin out adjacent observations and match the spatial resolution of the environmental variables.
We fitted a random forest classification algorithm using 1500 trees with the number of variables randomly sampled at each split (i.e. mtry parameter) set by default to 3 (Breiman, Reference Breiman2001). To evaluate the predictive accuracy of the model, a 10-fold cross validation method was adopted where each fold was comprised of independent presence-absence records for model training and testing. Folds were stratified by prevalence to ensure a similar proportion of presences and absences records across training and test datasets. For each fold, a random forest was fitted on training sets that correspond to 70% of available presence-absence records selected at random, and its performance was assessed using the remaining 30% of the records. Mean results across the 10 folds are presented hereafter. The relative importance of each explanatory variable and partial dependency plots were produced using the Caret package (Kuhn, Reference Kuhn2020).
Material examined
A specimen caught from the Celtic Sea during the 2021 Cefas Q1SWECOS survey was compared with the syntype material to confirm its identification and to examine any morphological differences that may be present among the North-east Atlantic specimen and the tropical Atlantic, African syntypes. The single male examined had a slightly larger carapace (1 mm wider and 0.8 mm longer) than that of the largest of the three syntype males but was otherwise morphologically compatible.
Type Material – Heterocrypta maltzami (Miers, Reference Miers1881)
Syntypes, 3 males (12.7 × 10.6 mm, 12.6 × 10.5 mm, 12.0 × 10.0 mm), 1 ovigerous female (12.1 × 10.5 mm), 1 female (10.4 × 9.1 mm) Natural History Museum London, NHM 1881.24, Goree Island, Senegal.
Present Material – Distolambrus maltzami (Miers, Reference Miers1881)
A single male. (13.7 × 11.4 mm). Collected 30 March 2021, located: 48°02.25′N 006°57.90′W, 164 m, Natural History Museum London, NHM UK 2022.915.
Synonymy
An extensive synonymy for the species is included to provide further insight into the rich nomenclatural history of Distolambrus maltzami which has been known by several specific names under its previous genus (Heterocrypta) before its current designation by Tan & Ng (Reference Tan and Ng2007).
Heterocrypta maltzami – Miers, Reference Miers1881: 209, 365, 374, pl. 13 fig. 1. – Balss, Reference Balss1921: 54. – Bouvier, Reference Bouvier1940: 315. – Manning & Holthuis, Reference Manning and Holthuis1981: 322. – García-Raso, Reference García-Raso1984: 108. – González-Gurriarán & Méndez, Reference González-Gurriarán and Méndez1985: 199, fig. 81, photo 63. – Fransen, Reference Fransen1991: 108. – Noël, Reference Noël1992: 127. – González-Pérez, Reference González-Pérez1995: 247. – Paulmier, Reference Paulmier1997: 32, pl. 49 fig. 3 – García-Raso, Reference García-Raso1996: 740. – Pipitone & Arculeo, Reference Pipitone and Arculeo2003: 77. – Henriksen, Reference Henriksen2009: 79, fig. 40. – Massi et al., Reference Massi, Micalizzi, Giusto and Pipitone2010: 1142, figs 2, 3.
Heterocrypta marionis – Milne-Edwards, Reference Milne-Edwards1881: 879. – Milne-Edwards, Reference Milne-Edwards1882: 38. – Perrier, Reference Perrier1886: 48, 299, fig. 17.
Heterocrypta marioni – Milne-Edwards & Bouvier, Reference Milne-Edwards and Bouvier1894: 23. – De Miranda y Rivera, Reference De Miranda y Rivera1921: 190.
Heterocrypta maltzani – Perrier, Reference Perrier1886: 299. – Ortmann, Reference Ortmann1894: 417. – Caullery, Reference Caullery and Koehler1896: 402. – Milne-Edwards and Bouvier, Reference Milne-Edwards and Bouvier1900: 121, pl. 19 fig. 6. – Rathbun, Reference Rathbun1900: 296. – De Miranda y Rivera, Reference De Miranda y Rivera1921: 190. – Sourie, Reference Sourie1954: 150. – Monod, Reference Monod1956: 589, figs 862–867. – Délye, Reference Délye1957: 3. – Longhurst, Reference Longhurst1958: 89. – Guinot & Ribeiro, Reference Guinot and Ribeiro1962: 80. – Rossignol, Reference Rossignol1962: 123. – Ribeiro, Reference Ribeiro1964: 21. – Forest & Guinot, Reference Forest and Guinot1966: 120. – Zariquiey-Álvarez, Reference Zariquiey-Álvarez1968: 442. – Maurin, Reference Maurin1968: 486. – Pastore, Reference Pastore1972: 106. – Lagardere, Reference Lagardere1973: 95. – Thiroit, Reference Thiroit1973: 112. – Türkay, Reference Türkay1975: 71, 74, fig. 6. – Anadón, Reference Anadón1981: 155. –Moncharmont, Reference Moncharmont1981: 107. – Noël, Reference Noël1983: 37. – Gauld, Reference Gauld1990: 72. – Števčić, Reference Števčić1990: 244. – Koukouras et al., Reference Koukouras, Dounas, Türkay and Voultsiadou-Koukoura1992: 225. –Serrano et al., Reference Serrano, Sánchez and García-Castrillo2006: 158.
Heterocrypta maltzani var. marioni – Milne-Edwards & Bouvier, Reference Milne-Edwards and Bouvier1900: 122, pl. 18 fig. 16, pl. 19 figs 1–5.
Heterocrypta maltzami var. marionis – Garcia & Gràcia, Reference Garcia and Gràcia1996: 181
Heterocrypta maltzami marionis – d'Udekem d'Acoz, Reference d'Udekem d'Acoz1999: 227.
Distolambrus maltzami – Voultsiadou et al., Reference Voultsiadou, Fryganiotis, Porra, Damianidis and Chintiroglou2011: 76. – Mura & Corda, Reference Mura and Corda2011: 679. – Brongiorni et al., Reference Brongiorni, Ravara, Parretti, Santos, Rodrigues, Amaro and Cunha2013: 80. –Marco-Herrero et al., Reference Marco-Herrero, Abelló, Drake, García-Raso, González-Gordillo, Guerao, Palero and Cuesta2015: 5.– Grimes et al., Reference Grimes, Bakalem and Dauvin2016: 390. – De Matos-Pita et al., Reference De Matos-Pita, Castillo and Ramil2017: 1292. – González et al., Reference González, Triay-Portella, Martins and Lopes2017: 143. – González, Reference González2018: 94. – Santin et al., Reference Santin, Aguilar, Akyol, Begburs, Benoit, Chimienti, Crocetta, Dalyan, De La Linde Rubio, Dragičević, Dulčić, Giglio, Gönülal, Kebapcioglu, Kesici, Kiparissis, Kousteni, Mancini, Mastrototaro, Menut, Montesanto, Peristeraki, Poursanidis, Renoult, Sánchez-Tocino, Sperone and Tiralongo2021: 211, fig. 13. – Massutí et al., Reference Massutí, Sánchez-Guillamón, Farriols, Palomino, Frank, Bárcenas, Rincón, Martínez-Carreño, Keller, López-Rodríguez and Díaz2021: 49.
Distribution
Eastern Atlantic: Celtic Sea (this study); Bay of Biscay (Milne-Edwards & Bouvier, Reference Milne-Edwards and Bouvier1894; Caullery, Reference Caullery and Koehler1896; Lagardere, Reference Lagardere1973); Cantabrian Sea (Serrano et al., Reference Serrano, Sánchez and García-Castrillo2006); Galician coast (González-Gurriarán & Méndez, Reference González-Gurriarán and Méndez1985); Azores (Miers, Reference Miers1881; Fransen, Reference Fransen1991); Canary Islands (Fransen, Reference Fransen1991; González-Pérez, Reference González-Pérez1995; González, Reference González2018); Cape Verde Islands (Milne-Edwards & Bouvier, Reference Milne-Edwards and Bouvier1900; Fransen, Reference Fransen1991); off Western Sahara (Anadón, Reference Anadón1981); Mauritania (Anadón, Reference Anadón1981; Fransen, Reference Fransen1991; De Matos-Pita et al., Reference De Matos-Pita, Castillo and Ramil2017); Gulf of Guinea (Henriksen, Reference Henriksen2009); off Republic of the Congo (Balss, Reference Balss1921; Rossignol, Reference Rossignol1962). Seemingly no confirmed records from Portugal (Nobre, Reference Nobre1931; Marco-Herrero et al., Reference Marco-Herrero, Abelló, Drake, García-Raso, González-Gordillo, Guerao, Palero and Cuesta2015), though it has been found in adjacent waters to the north and south.
Mediterranean: Málaga, Southern Spain (García-Raso, Reference García-Raso1984; García-Raso et al., Reference García-Raso, González-Gurriarán and Sardá1987); Alboran Sea, off Morocco (García-Raso, Reference García-Raso1984); Habibas Islands, off Algeria (Délye, Reference Délye1957); Balearic Sea (De Miranda y Rivera, Reference De Miranda y Rivera1933); Balearic Islands (Garcia & Gràcia, Reference Garcia and Gràcia1996; Massutí et al., Reference Massutí, Sánchez-Guillamón, Farriols, Palomino, Frank, Bárcenas, Rincón, Martínez-Carreño, Keller, López-Rodríguez and Díaz2021); Banyuls (Noël, Reference Noël1983); Cap Sicié (Milne-Edwards & Bouvier, Reference Milne-Edwards and Bouvier1900); Gulf of Naples (Moncharmont, Reference Moncharmont1981); Straits of Sicily (Massi et al., Reference Massi, Micalizzi, Giusto and Pipitone2010); Adriatic Sea (Pesta, Reference Pesta1918; Števčić, Reference Števčić1990); Ionian Sea (Pastore, Reference Pastore1972); Aegean Sea (Koukouras et al., Reference Koukouras, Dounas, Türkay and Voultsiadou-Koukoura1992; Voultsiadou et al., Reference Voultsiadou, Fryganiotis, Porra, Damianidis and Chintiroglou2011; Santin et al., Reference Santin, Aguilar, Akyol, Begburs, Benoit, Chimienti, Crocetta, Dalyan, De La Linde Rubio, Dragičević, Dulčić, Giglio, Gönülal, Kebapcioglu, Kesici, Kiparissis, Kousteni, Mancini, Mastrototaro, Menut, Montesanto, Peristeraki, Poursanidis, Renoult, Sánchez-Tocino, Sperone and Tiralongo2021).
Results
Since the start of comprehensive epi-faunal identification and enumeration of benthic catches sampled during the EVHOE and the Q1SWECOS survey programmes a total of 73 specimens of D. maltzami has been recorded (Figure 1). The specimens have predominately been collected from the Bay of Biscay, at depths of 100–250 m, along the continental shelf edge although several records were observed in the southern part of the Celtic Sea area. When observed, generally only single specimens were found with only a single individual recorded in 67% of the trawls where it was caught. This pattern of catching lone individuals is typical for the species, based on other studies (e.g. Massi et al., Reference Massi, Micalizzi, Giusto and Pipitone2010; Voultsiadou et al., Reference Voultsiadou, Fryganiotis, Porra, Damianidis and Chintiroglou2011; Santin et al., Reference Santin, Aguilar, Akyol, Begburs, Benoit, Chimienti, Crocetta, Dalyan, De La Linde Rubio, Dragičević, Dulčić, Giglio, Gönülal, Kebapcioglu, Kesici, Kiparissis, Kousteni, Mancini, Mastrototaro, Menut, Montesanto, Peristeraki, Poursanidis, Renoult, Sánchez-Tocino, Sperone and Tiralongo2021).
During a EVHOE survey, on 15 November 2014 a single specimen of Distolambrus maltzami was captured at 48°45′51.8″N 008°14′15.4″W from a depth of 161 m. This represents the first UK record of D. maltzami and currently its most northerly reported occurrence. The crab has more recently been observed in the same area in 2021 during the Q1SWECOS survey. Before these survey series, only 5 individual records had been recorded within the Bay of Biscay from a depth of 180 m (Milne-Edwards & Bouvier, Reference Milne-Edwards and Bouvier1894; Lagardere, Reference Lagardere1973; Paulmier, Reference Paulmier1997).
Species distribution modelling
The 10-fold cross-validation yielded a mean estimate of the Area Under the Curve (AUC) of 0.84, which suggests that the model was good at discriminating between presence/absence of Distolambrus maltzami in the test datasets. A set of eight environmental variables related to ocean physical (e.g. current, temperature) and biogeochemical conditions (e.g. chlorophyll-a, dissolved oxygen, nitrate concentration) contributed to a relatively similar level of model prediction accuracy, and characterized suitable environmental conditions for the species (Figure 2). Partial influence plots, which describe where the species is most likely to occur, were created with the four most influential predictors, namely: standard deviation of current velocity (Figure 3A), maximum chlorophyll-a concentrations (Figure 3B), minimum bottom temperature (Figure 3C), and dissolved oxygen concentrations (Figure 3D). These variables characterize the water mass properties where D. maltzami is most likely to occur, i.e. at depth along the continental shelf (Figure 4). For instance, the probability of occurrence was greatest in areas exposed to fluctuating tidal currents (Figure 3A), and where chlorophyll-a-rich (Figure 3B), cold (minimum sea-bed temperature <10°C; Figure 3C) and oxygen-rich water (Figure 3D) masses occur. Given model AUC, the map of predicted probability of the presence of D. maltzami can be interpreted as an accurate broad-scale description of the potential occurrence of D. maltzami in the Celtic Sea/Bay of Biscay area (Figure 4).

Fig. 2. Environmental variable importance (based on decrease in model accuracy) to predictive modelling of the distribution of the crab D. maltzami.

Fig. 3. Partial influence of (A) standard deviation of current velocity, (B) maximum chlorophyll-a concentration, (C) minimum bottom temperature and (D) dissolved oxygen concentration plotted against the probability of D. maltzami where P is the probability of presence.

Fig. 4. Predicted probability of presence of the crab D. maltzami based on EVHOE data between 2008 and 2018 (1460 stations).
Identifying Distolambrus maltzami
Distolambrus maltzami is readily distinguished from other species and genera within the Parthenopidae due to: its uniquely shaped pentagonal carapace and smooth dorsal surface and presence of a V-shaped ridge on the gastric region (centre of the dorsal carapace); a largely entire marginal edge and expanded posterior part of the carapace and partially covering the ambulatory legs; and the posterior margin entire, without protrusions or teeth-like structures (Figure 5A–5D) (Tan & Ng, Reference Tan and Ng2007). However, to assist correct identification, should examples of Parthenopidae species be encountered from fisheries surveys within the North-east Atlantic, a simplified key is provided.
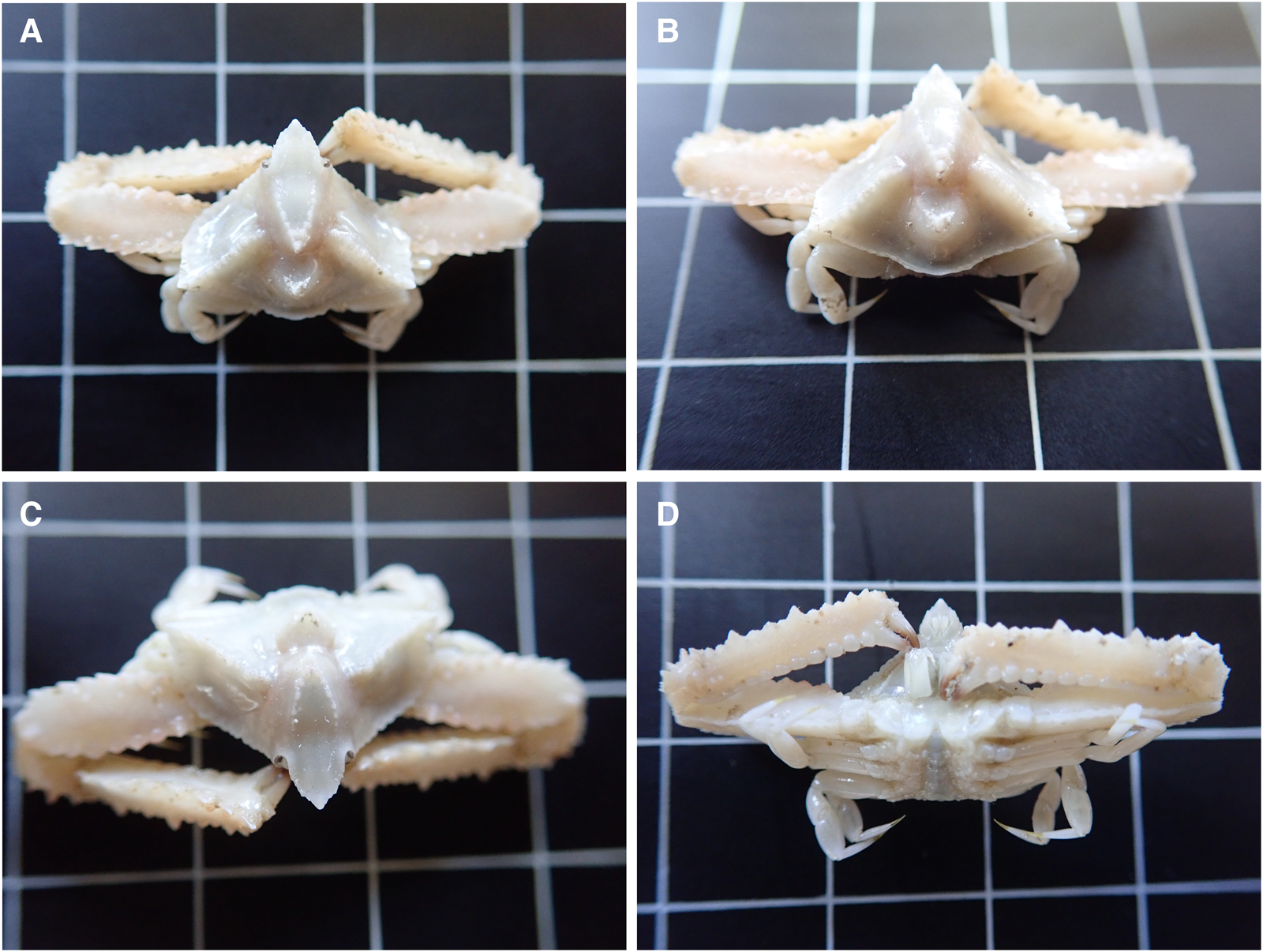
Fig. 5. Distolambrus maltzami captured during the Q1SWECOS survey (2021) showing (A) dorsal view of the carapace, (B) dorsal view of the posterior of the carapace displaying the pronounced cardiac region, (C) frontal view of rostrum and (D) ventral view displaying mouthparts and epistome. Grid squares are 10 mm × 10 mm.
Ingle (Reference Ingle1980), in his book on British crabs, included Parthenopoides massena (Roux, Reference Roux1830) (Figure 3A) which has the potential to be confused with D. maltzami particularly during onboard sorting of epi-faunal catch. Ingle (Reference Ingle1980) noted P. massena has ‘their northernmost limits at about the Ushant area’, in France which is ~1° south of UK territorial waters. As such, one may reasonably expect that P. massena occurs in UK waters. Indeed, Clark (Reference Clark1986) reported observations of P. massena from the French sector of the western Channel, and P. massena has, to date, been the only member of the family Parthenopidae listed for the English Channel (Hayward & Ryland, Reference Hayward and Ryland1990; Holmes et al., Reference Holmes, Costello, Connor, Howson and Picton1997). Hence, a simplified key for the differentiation of D. maltzami (Figure 6B) from the superficially similar P. massenai (Figure 6A) is provided here.

Fig. 6. (A) Parthenopoides massena (Roux, Reference Roux1830); male, 16.0 mm × 15.6 mm (USNM 14507), Naples, Italy and (B) specimen of D. maltzami; male 13.7 × 11.4 mm for comparison.
Key to Distolambrus maltzami and Parthenopoides massena
-
Carapace sub-triangular (Figure 6A), slightly longer than broad; dorsal surface tuberculate. Carapace not expanded, especially near the posterior region, and ambulatory legs are largely exposed. Exorbital region with a distinct constriction, forming a tooth-like structure. Gastric region not clearly differentiated, but possesses a raised blunt tooth of varying height……Parthenopoides massena (Roux, Reference Roux1830)
-
Carapace pentagonal in shape (Figure 6B), slightly broader than long; dorsal surface generally smooth except for distinct ridges on the gastric, epibranchial and cardiac regions. Carapace lateral margins expanded, partially covering ambulatory legs. Exorbital region without a distinct constriction. Gastric region with a distinct V-shaped ridge……Distolambrus maltzami (Miers, Reference Miers1881)
Discussion
The relative paucity of records of Distolambrus maltzami implies that it is unlikely to be locally abundant in areas where it does occur and so D. maltzami can be considered an uncommon species (Gönülal in Santin et al., Reference Santin, Aguilar, Akyol, Begburs, Benoit, Chimienti, Crocetta, Dalyan, De La Linde Rubio, Dragičević, Dulčić, Giglio, Gönülal, Kebapcioglu, Kesici, Kiparissis, Kousteni, Mancini, Mastrototaro, Menut, Montesanto, Peristeraki, Poursanidis, Renoult, Sánchez-Tocino, Sperone and Tiralongo2021). Catchability of small epifaunal species in beam trawls is relatively low (Reiss et al., Reference Reiss, Kröncke and Ehrich2006), which is most likely the case for D. maltzami (<15 mm carapace width) in survey trawls with a liner of 20 mm (EVHOE) and 40 mm (Q1SWECOS) such as those used in this study, but it is probable that populations exist in low abundance across its range. Individuals may get recorded sporadically when they are captured, for example when the mesh liner is restricted by the catch such as turf fauna. For example, a thorough sort of megabenthos collected using a 5 mm mesh liner recovered 43 individuals of D. maltzami from across 85 trawls of the Balearic Islands (Massutí et al., Reference Massutí, Sánchez-Guillamón, Farriols, Palomino, Frank, Bárcenas, Rincón, Martínez-Carreño, Keller, López-Rodríguez and Díaz2021). Additionally, there is comparatively less sampling effort on the shelf edge habitats where it occurs, compared with shallower areas of the inner continental shelf. A combination of these factors may help explain the apparent lack of records of D. maltzami in the North-east Atlantic to date, such as the paucity of records off Portugal where we expect it to occur but it has not been observed nor recorded (Marco-Herrero et al., Reference Marco-Herrero, Abelló, Drake, García-Raso, González-Gordillo, Guerao, Palero and Cuesta2015). Thus, the records presented here can be considered the most accurate representation of the current detectable distribution of D. maltzami within the Bay of Biscay and Celtic Sea region without further changes to trawling gear or expansion of sampling effort.
The addition of species distribution modelling complements local observation records to characterize the expected distribution of D. maltzami along the edge of the continental shelf between latitude 45–49°N (Figures 4). While not yet observed, the model indicates that the species may extend to south-west Ireland and surveys on the Porcupine Bank and off Ireland should make efforts to detect this species. The results of our study also suggest that granulometry and/or fine-scale local conditions had a limited contribution to model predictions, although this is based on low resolution maps with only a few EUNIS categories. Therefore, any habitat associations with granulometry need further investigation but it has been reported on a variety of substrate types from coarse to fine sediment (Pesta, Reference Pesta1918; Manning & Holthuis, Reference Manning and Holthuis1981). In contrast, deep continental shelf water mass properties contributed more to the determination of the modelled species niche, i.e. the range of oceanic conditions where the species is more likely to occur. Of particular interest is our finding that D. maltzami was found in waters where annual minimum temperatures were generally lower than 10°C. This contrasts with many records of more southerly specimens, with Mediterranean and West African records occurring in warmer waters between the sublittoral to 100 m depth (Manning & Holthuis, Reference Manning and Holthuis1981; Massi et al., Reference Massi, Micalizzi, Giusto and Pipitone2010; Santin et al., Reference Santin, Aguilar, Akyol, Begburs, Benoit, Chimienti, Crocetta, Dalyan, De La Linde Rubio, Dragičević, Dulčić, Giglio, Gönülal, Kebapcioglu, Kesici, Kiparissis, Kousteni, Mancini, Mastrototaro, Menut, Montesanto, Peristeraki, Poursanidis, Renoult, Sánchez-Tocino, Sperone and Tiralongo2021). This may suggest some correlation between the influencing factors we identified, such as minimum bottom temperature, current velocity or dissolved oxygen which appear to drive the distribution. Alternatively, the Bay of Biscay, Mediterranean and West African populations may have divergent favourable environmental conditions yet to be identified.
This paper describes a comprehensive overview of the spatial distribution of D. maltzami with a focus on the North-east Atlantic and highlights the importance of employing a multidisciplinary approach to fisheries data collection surveys such as the inclusion of thorough benthic monitoring in various programmes and of deploying appropriate survey equipment (particularly a fine mesh) to enable the most complete assessment of benthic assemblages. Combining multiple records with distribution modelling results in a stronger basis to better determine and monitor the distribution of rare macroinvertebrates and affords future researchers the possibility to detect changes in their distribution or abundance that might occur over time either directly as a result of climatic changes or indirectly from any alteration of inter- and intra-specific interactions in the benthic assemblage.
Data
The datasets from the surveys are openly available in the ICES DATRAS repository, Datras: Download (ices.dk) or from the IFREMER/HARMONIE database. The specimen caught during the 2021 Q1SWECOS survey and compared with the syntype material has been deposited with the Natural History Museum under the BOLD project.
Acknowledgements
We thank the scientists and crew of the research vessels for their help during field surveys, Jim Ellis (CEFAS) and the anonymous reviewers for providing comments on the manuscript.
Authors contributions
H.C. wrote the manuscript along with P.B. and P.M. S.T. provided confirmation of the species identification and detailed descriptions of key identifying features. B.F. and M.M. created the model for predicted species distribution. All authors read and approved the final manuscript.
Financial support
The CEFAS survey series is funded by DEFRA under the Fisheries and Aquaculture Service Level Agreement (SLA) and investigation into the species was funded internally through CEFAS. The French surveys series is funded through the European Union Data Collection Framework (DCF) and the Data collection MultiAnnual Programme (DCMAP).
Conflict of interest
The authors declare none.
Ethical standards
Not applicable.








