Introduction
The common bottlenose dolphin (Tursiops truncatus) (Montagu, 1821) is often considered to be a ‘cosmopolitan’ species in the world's oceans (Pilleri & Gihr, Reference Pilleri and Gihr1969) and has a worldwide coastal and offshore distribution within both tropical and temperate waters (Bearzi & Fortuna, Reference Bearzi, Fortuna, Reeves and Notarbartolo di Sciara2006). Due to the overlapping presence of bottlenose dolphins and human activities in coastal zones, they are confronted with the negative consequences of a multitude of human impacts (Bulleri & Chapman, Reference Bulleri and Chapman2010). Historical intentional killing, incidental mortalities due to various (and often destructive) fishing practices, fish stock depletion and habitat degradation are listed as the main contributors to population decline (Bearzi et al., Reference Bearzi, Fortuna and Reeves2012). In fact, it has been estimated that there are now fewer than 10,000 bottlenose dolphins present within the Mediterranean Sea (Bearzi et al., Reference Bearzi, Fortuna and Reeves2012), with a population decline of ~30% over the past 60 years (Bearzi et al., Reference Bearzi, Agazzi, Bonizzoni, Costa and Azzellino2008). While the Mediterranean subpopulation of bottlenose dolphins is classified as ‘vulnerable’, the Black Sea subspecies T. truncatus ponticus is classified as ‘endangered’ (Birkun, Reference Birkun2012). Although the total population size within the Black Sea remains unknown, the present population is believed to be at least several thousand animals (Reeves & Notarbartolo Di Sciara, Reference Reeves and Notarbartolo Di Sciara2006; Birkun, Reference Birkun2012). The species is listed within Annex II and IV of the EU Habitats Directive (92/43/EEC) as a species of ‘community interest whose conservation requires both designation of special areas and strict protection’ (Natura 2000 Network).
The Istanbul Strait is well known for its cultural, strategic and economic importance. It is the only waterway connecting the Black Sea and Marmara Sea. It is one of the narrowest and busiest straits in the world with a minimum distance of 698 m between the European and Asian coasts (Özsoy et al., Reference Özsoy, Çagatay, Balkıs and Öztürk2016). The annual number of marine vessels travelling through the Istanbul Strait was ~4500 in 1936. This figure had increased dramatically to 45,529 marine vessels passing through the strait in 2014 (Kara, Reference Kara2016). The average number of cargo ships is estimated to be around 130 per day, while daily local traffic accounts for ~1200 vessels (Directorate General of Coastal Safety, 2014). In addition, fishery is an important industry in Turkey, with the Istanbul Strait being the second most important fishery region after the East Black Sea Region (TUIK, 2016). Both small-scale artisanal fisheries and large-scale commercial fisheries are active in the area. Thirteen fishery cooperatives and 17 fishing ports are located in the strait, with 14 of the 17 ports located in the north section alone (Öztürk et al., Reference Öztürk, Karakulak, Öztürk, Oral and Öztürk2006). While artisanal fishery is utilized throughout the strait all year round, the northern sections are subjected to intense purse seining from September to mid-April. Although the current study was not specifically focused on fisheries, substantial numbers of commercial fishing vessels were recorded with an average of 100 vessels daily, during autumn and winter in the middle and north sections. Sometimes more than 50 vessels could be sighted at a time (Baş et al., Reference Baş, Öztürk and Öztürk2015).
In addition to its great economic value, this body of water also acts as a biological corridor, a biological barrier, and an acclimatization zone for migrating species, including dolphins, porpoises and migratory fishes, allowing the species to adjust to the different environmental conditions between the Mediterranean and Black Sea, and vice versa (Öztürk & Öztürk, Reference Öztürk and Öztürk1996). The regular presence of bottlenose dolphins within Turkish territorial waters has been recorded since the last half century (Marchessaux, Reference Marchessaux1980; Çelikkale et al., Reference Çelikkale, Ünsal, Durukanoğlu, Karaçam and Düzgüneş1988; Öztürk & Öztürk, Reference Öztürk, Öztürk, Evans, Parsons and Clark1997; Dede, Reference Dede1999; Dede & Öztürk, Reference Dede and Öztürk2007; Dede et al., Reference Dede, Öztürk and Tonay2008; Öztürk et al., Reference Öztürk, Dede and Tonay2009; Dede & Tonay, Reference Dede and Tonay2010; Dede et al., Reference Dede, Saad, Fakhri and Öztürk2012; Baş et al., Reference Baş, Öztürk and Öztürk2015). Despite the established population decline over recent decades in the Mediterranean Basin, only in recent years have a few dedicated surveys been conducted, producing information on abundance estimates, encounter rates, residency patterns and site fidelities within the Eastern Mediterranean Sea and Black Sea (Birkun et al., Reference Birkun, Northridge, Willsteed, James, Kilgour, Lander and Fitzgerald2014; Gladilina & Gol'din, Reference Gladilina and Gol'din2014, Reference Gladilina and Gol'din2016; Ryan et al., Reference Ryan, Cucknell, Romagosa, Boisseau, Moscrop, Frantzis and McLanaghan2014; Baş et al., Reference Baş, Öztürk and Öztürk2015). The lack of baseline data is one of the main barriers on the effort to accurately direct conservation and management strategies towards the mitigation of anthropogenic impacts (Balmer et al., Reference Balmer, Schwacke, Wells, Adams, George, Lane, Mclellan, Rosel, Sparks, Speakmen, Zolman and Pabst2013).
Within Turkish waters, a study conducted in 1987 reported the presence of 11,213 bottlenose dolphins within the Turkish Black Sea and an encounter rate of 1.09 individuals per km2 for all cetacean species (Çelikkale et al., Reference Çelikkale, Ünsal, Durukanoğlu, Karaçam and Düzgüneş1988), although such results were subject to criticism due to the methodology adopted (IWC, 1992; Birkun, Reference Birkun and Notarbartolo di Sciara2002). In 1998, it was estimated that there were 468 bottlenose dolphins within the Turkish Straits System (Dede, Reference Dede1999). In 2008, the average encounter rate of cetaceans within the Istanbul Strait was reported as 0.76 sightings per 10 km (0.143 sightings per nmile) (Öztürk et al., Reference Öztürk, Dede and Tonay2009), whereas this rate was 0.27 and 0.23 groups per 10 km in the Marmara Sea and the North Aegean Sea, respectively (Altuğ et al., Reference Altuğ, Altan, Oral, Topaloğlu, Dede, Kesin, İşinibilir, Çardak and Çiftçi2011). Finally, Ryan et al. (Reference Ryan, Cucknell, Romagosa, Boisseau, Moscrop, Frantzis and McLanaghan2014) reported an encounter rate for bottlenose dolphins in the Aegean Sea as 0.06 groups per 10 km. However, the majority of the above studies were neither species-specific nor employed the same methodology.
In this study, we conducted boat-based surveys between 2011 and 2013 to build the first photo-identification catalogue of bottlenose dolphins in the Istanbul Strait. Data were used to investigate encounter rates, residency patterns and site fidelities in order to understand the spatial and temporal distribution of bottlenose dolphins and ultimately provide the basis for effective conservation and management decisions.
Materials and methods
Study area
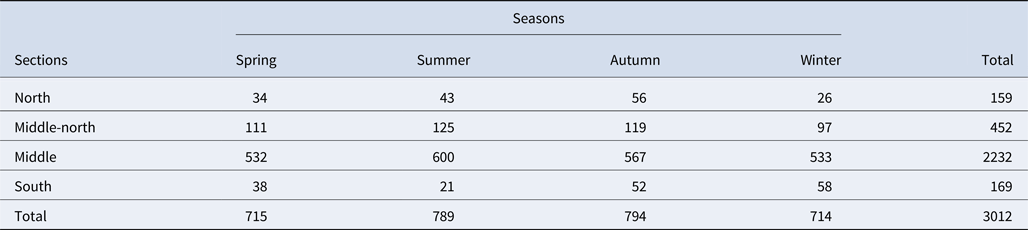
The study area includes the Istanbul Strait and adjacent waters of the Black Sea and Marmara Sea. The study area was divided into four sections (North, Middle-North, Middle and South) based on geographic location, vessel density and traffic patterns (Figure 1; Table 1).
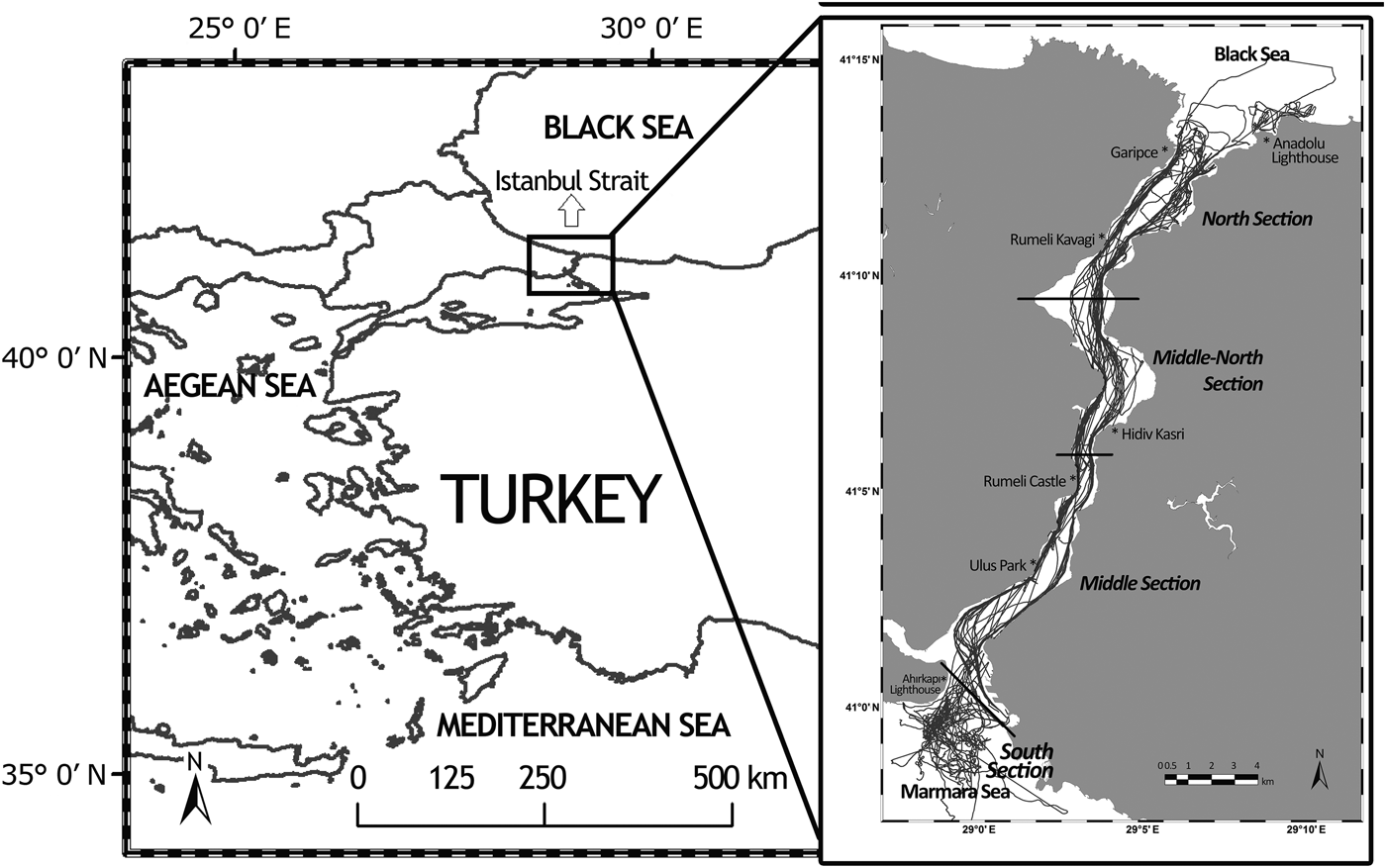
Fig. 1. The survey area. Black lines in the sea represent the followed boat routes throughout the study. Red lines represent the borders of each selected section.
Table 1. Daily average of marine vessel presence in each section and season (numbers taken from Baş Reference Baş2014)
Data collection
Dedicated monthly boat-based surveys were conducted using a 16 m gullet boat with a 185 horsepower engine between 16 July 2011 and 20 December 2013 to collect bottlenose dolphin photo identification data. This well-known method uses natural markings on the dorsal fin to identify individual dolphins (Würsig & Würsig, Reference Würsig and Würsig1977). A minimum of two surveys, with an average length of 5 h per survey, were conducted each month over the course of 29 months. Boat routes covered both the European and Asian coast of the strait and followed one of three routes: the entire strait; south section including the adjacent waters of the Marmara Sea; north section including the adjacent waters of the Black Sea (Figure 1). The software Logger 2010, Version 5 © (developed by IFAW; http://www.ifaw.org) was continuously used during the surveys to record the research boat track line as well as the distance of the sighted group from the research boat and their angle from the north.
For this particular study, a ‘group’ was defined as a collection of individuals engaging in similar behaviours, with close-group cohesion (less than 50 m). A focal group was typically followed at a distance of 50–400 m, but if an individual approached the research boat, the speed was gradually reduced until idle speed was reached, and any sudden movements were avoided in order to minimize our impact.
Photo-identification data were collected by a minimum of two photographers positioned on the bow of the research boat. Both photographers used a Nikon D 80 with 70–300 mm lens generally from a distance of 10–300 m. In order to ensure all individuals within the group were photographed, an attempt was made to capture numerous pictures of the individuals in the group, with particular care taken to avoid bias toward the more distinctive individuals. When possible both sides of the individual were photographed.
Photographs were processed in three stages: storage, cataloguing and matching. During storage, photographs were cropped around the fin and the body for each individual in Adobe Photoshop CS5. Later they were stored according to quality rating: (i) Good quality (dorsal fin is focused, perpendicular to the camera and the entire fin is in the frame); (ii) Medium quality (dorsal fin is focused with a satisfactory angle and the entire fin is in the frame); (iii) Bad quality (dorsal fin is out of focus and/or the entire fin is not in the frame); (iv) No info (photograph doesn't hold any photo-identification value). The cataloguing stage included only Good and Medium quality photographs, and was carried out using Imatch Database, Version 4. Individuals were catalogued according to markings (nicks and scars) on the dorsal fin, fin shape, and body deformities (Würsig & Würsig, Reference Würsig and Würsig1977; Würsig & Jefferson, Reference Würsig and Jefferson1990). Later, each individual was classified into one of four categories of distinctiveness: Low, Medium, Good or Distinguished. ‘Low’ individuals had no identifiable features/marks, ‘Medium’ individuals had small nick(s) or scar(s) that were difficult to re-identify, ‘Good’ individuals had both several nicks and scars in the same frame, instead of holding a single small nick or a scar, and ‘Distinguished’ individuals had at least one permanent, clear and easily identifiable notch. Thus, individual distinctiveness did not refer to image sharpness but rather to the presence or absence of recognizable features on each individual. The final stage, matching, included individuals from the ‘Distinguished’ and ‘Good’ distinctiveness category. To avoid misidentification, calves and individuals without distinctive markings were not included in analysis. Furthermore, to avoid overestimating residency pattern and site fidelity, individuals re-sighted in the same day were only recorded once and re-sightings were excluded from the analysis.
Additionally, opportunistic data were collected from dolphin watch boats and local ferries by a wildlife photographer (Yunus Arakon) from 2014 to 2016, who reported the sightings and their location, with accompanying photographs. The opportunistic sighting data were only used in residency pattern and site fidelity analysis and the photos were processed according to their quality and distinctiveness as explained above.
Data analysis
Survey effort
To test if the survey effort and days of dolphin sightings were evenly distributed for each season, a Chi-square test was conducted. To evaluate whether all sighted individuals were photographed and identified, a one-way ANOVA test was used to compare the group size estimated visually with the number of individuals photographed in each group. Statistical Package, SPSS® (Version 20), was used to carry out the analysis.
Encounter rates
Group and individual encounter rates were calculated both for the entire study period and for each season, using a methodology explained previously by Bearzi et al. (Reference Bearzi, Agazzi, Bonizzoni, Costa and Azzellino2008). Seasons were classified as autumn (September, October, November), winter (December, January, February), spring (March, April, May) and summer (June, July, August). Encounter rate per km was calculated as n/L, where n represents the total number of sighted groups or individuals and L represents the total transect length in km (Bearzi et al., Reference Bearzi, Agazzi, Bonizzoni, Costa and Azzellino2008). Data were prepared, visualized and analysed in ESRI ArcGIS software Version 9.3. Prior to analysis, the study area was divided into cells of 500 m × 500 m to normalize the data collected during the uneven survey efforts. Cells with a survey effort lower than the cell's diagonal (707 m) were excluded from the analysis. As several cells contained variable portions of land, encounter rates within such cells were weighed against the relative proportion of land within the cell:
Weight estimators were then used in each step for all cells.
Residency pattern and site fidelity
Photographed individuals were classified according to their sighting history in each month. The mean and the maximum number of re-sightings for the entire catalogue were reported as the descriptive statistic. Residency pattern was defined as the tendency of an individual to remain in or return to a study area (Daly et al., Reference Daly, Smale, Cowley and Froneman2014). Monthly residency rate (the number of months a dolphin was sighted as a proportion of the total number of months surveyed) and seasonal residency rate (the number of seasons a dolphin was sighted as a proportion of the total number of seasons surveyed) were calculated. Additionally, site fidelity (re-use of the study area) was calculated for each identified dolphin by calculating the ratio between the number of sightings and number of survey days from an individual's first sighting to its last re-sighting (Daly et al., Reference Daly, Smale, Cowley and Froneman2014; Zanardo, Reference Zanardo2016). A site fidelity value of one would indicate that the dolphin was photographed on every single survey day.
An agglomerative hierarchical cluster analysis was performed via XLSTAT 2017® (Addinsoft, Paris, France) in order to distinguish groups or ‘clusters’ of individuals with a similar degree of monthly residency, seasonal residency and site fidelity indices (Zanardo, Reference Zanardo2016). Agglomerative hierarchical clustering is a bottom-up clustering method that starts with each observation as an individual cluster, the clusters are then combined based on similarity until all clusters have been combined into one (Zanardo, Reference Zanardo2016). Squared Euclidean distance was chosen for the dissimilarity measure and Ward's method as the clustering algorithm. Automatic truncation was selected for the dissimilarity threshold, and results were displayed as a dendrogram. To check the validity of the dendrogram, the cophenetic correlation coefficient (CCC) was calculated using StatistiXL® (Version 2, Nedlands, Western Australia). When the CCC is close to 1, the dendrogram represents more accurately how the clustering solution reflects the data structure (Zanardo, Reference Zanardo2016).
Results
Survey effort
Overall, 58 days (297 h) of boat-based surveys were conducted between 2011 and 2013. Bottlenose dolphins were sighted on 49 days (61 h), involving 360 group encounters. Survey effort was statistically constant throughout the seasons (χ2 = 3.103, df = 3, P = 0.37) and number of days that dolphins were sighted did not show a significant difference between seasons (χ2 = 2.837, df = 3, P = 0.42) (Table 2). Opportunistic data were collected over 7 days between 2014 and 2016.
Table 2. Days (hours) of survey effort under three boat routes for each season (Effort represents total survey effort for each season and square brackets represent days of dolphin sightings)
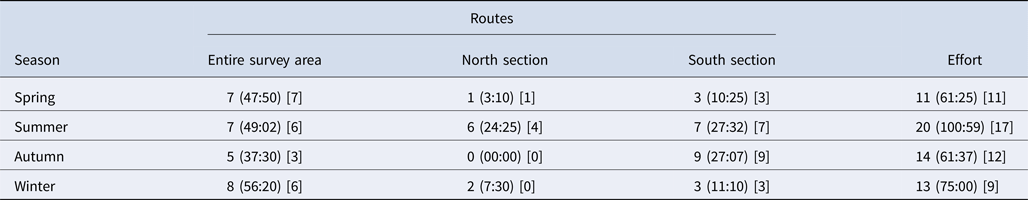
Photo identification
Over 6000 pictures were analysed through Imatch Database, Version 4©. Based on good and medium quality photographs, a total of 87 individual dolphins were identified (Supplementary Material 1). Of these, 11 individuals were photographed only in one month (13%), whereas the rest were re-sighted in at least two different months. Out of the 76 re-sighted individuals, 60 were recorded in at least two different years. The mean number of re-sightings was 3.4 (±1.9), with a maximum of 10 re-sightings. Additionally, 43 identified individuals were re-sighted in both entries of the strait, with the maximum re-sighting distance being 35 km.
Despite the effort to capture all the individuals in the group, considerable numbers of unmarked dorsal fins in combination with the bad quality photographs made it difficult to identify all sighted individuals. As a result, the number of photo-identified individuals in a group was significantly lower than the sighted group size (F = 22.530, df = 1, P < 0.0001).
Encounter rate
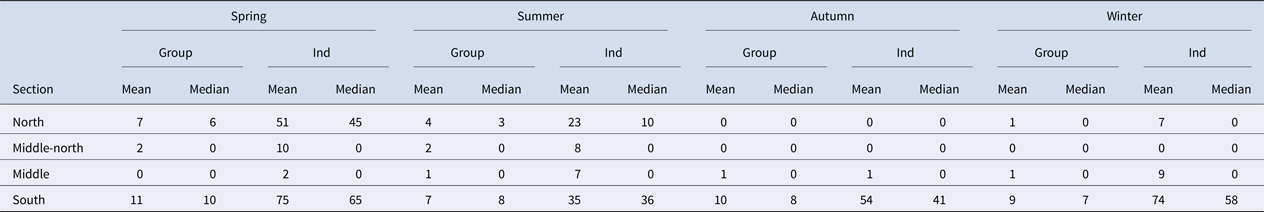
A total of 337 grid cells present within the survey area were analysed, and 270 of these cells were considered for the encounter rate analysis. The encounter rate of bottlenose dolphins was an average of four groups (average of 22 individuals) per 10 km within the Istanbul Strait. However, the encounter rates varied between seasons and geographic sections. Spring had the highest encounter rate with an average of five groups (average of 36 individuals) per 10 km throughout the strait. The south section had the highest encounter rate with an average of nine groups (average of 53 individuals) per 10 km throughout the year (Table 3). Conversely, the middle and middle-north sections had relatively low encounter rates throughout the year, with an average of one group per 10 km. The middle-north section had no sightings during autumn or winter. The same pattern was recorded for the north section in autumn. This area showed the highest number of encounters in spring (seven groups and 51 individuals per 10 km), followed by summer (four groups and 23 individuals per 10 km) (Table 3).
Table 3. Mean and median group and individual encounter rates per 10 km within the survey area
Residency pattern and site fidelity
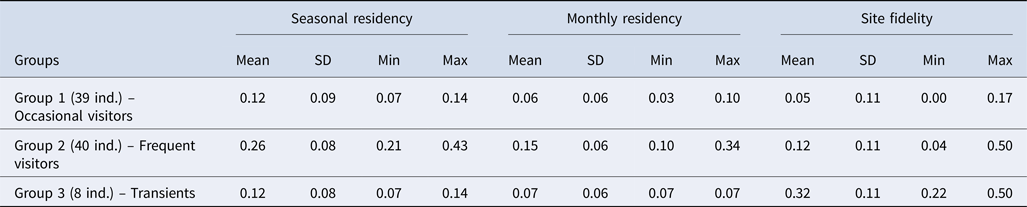
Eighty-seven identified individuals were considered in the residency pattern and site fidelity analysis. Monthly residency rate of bottlenose dolphins within the Istanbul Strait ranged from 0.03 (sighted only 1 month) to 0.34 (sighted up to 11 months), with a mean of 0.10 ± 0.06. Seasonal residency rate ranged from 0.07 (sighted during one season) to 0.43 (sighted up to six seasons), with a mean of 0.19 ± 0.09. Site fidelity index was on average 0.11 ± 0.10, ranging from 0 to 0.50.
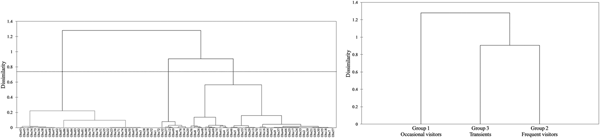
Regarding the hierarchical cluster analysis, three main groups of bottlenose dolphins were identified within the Istanbul Strait (Table 4). CCC was 0.68, therefore the dendrogram was a reasonable representation of the dissimilarities amongst the groups (Figures 2 and 3). Group 1 was composed of 39 individuals sighted on 1–3 different months, with low site fidelity indices ranging from 0.00–0.17. The individuals re-sighted showed a multi-year presence with an intermediate seasonal residency (0.12), low monthly residency (0.06), low site fidelity (0.05) and prolonged periods of absence; they were thus classified as ‘occasional visitors’ to the study site (Figures 2 and 3). Group 2 had 40 individuals sighted up to 11 months and exhibited relatively high seasonal (0.26) and monthly (0.15) residencies, with an intermediate site fidelity (0.12) and short periods of absence, they were thus classified as ‘frequent visitors’. Lastly, Group 3 consisted of eight individuals that exhibited relatively high site fidelity (0.32) due to their sightings in continuous months in a single year. Group 3 individuals were re-sighted 1–2 months, thus had a relatively low monthly (0.07) and intermediate seasonal residency (0.12), and were classified as ‘transients’. Regarding their re-sighting distance, 16 individuals of Group 1, 23 individuals of Group 2 and four individuals of Group 3 were re-sighted both in the southern and northern parts of the strait, with a maximum linear distance of 35 km (Figures 2 and 3).
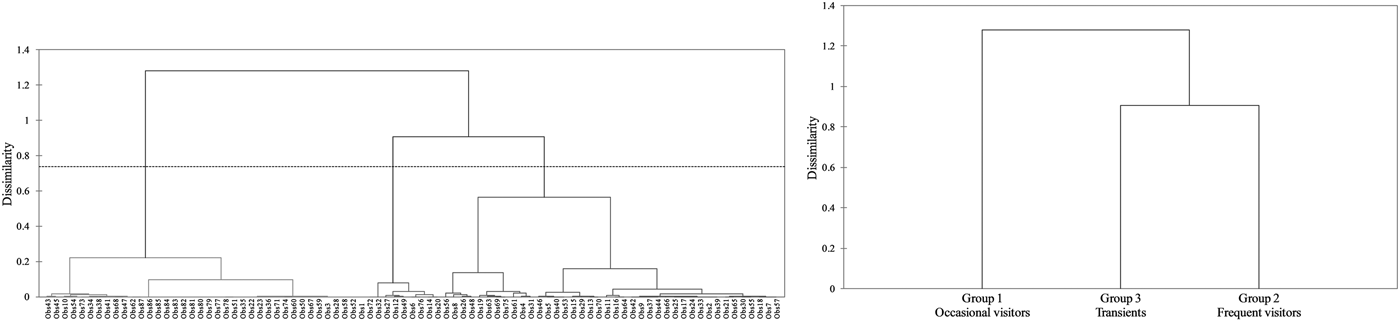
Fig. 2. The dendrogram of the agglomerative hierarchical clustering analysis resulted in three clusters. The dashed line represents the dissimilarity threshold.
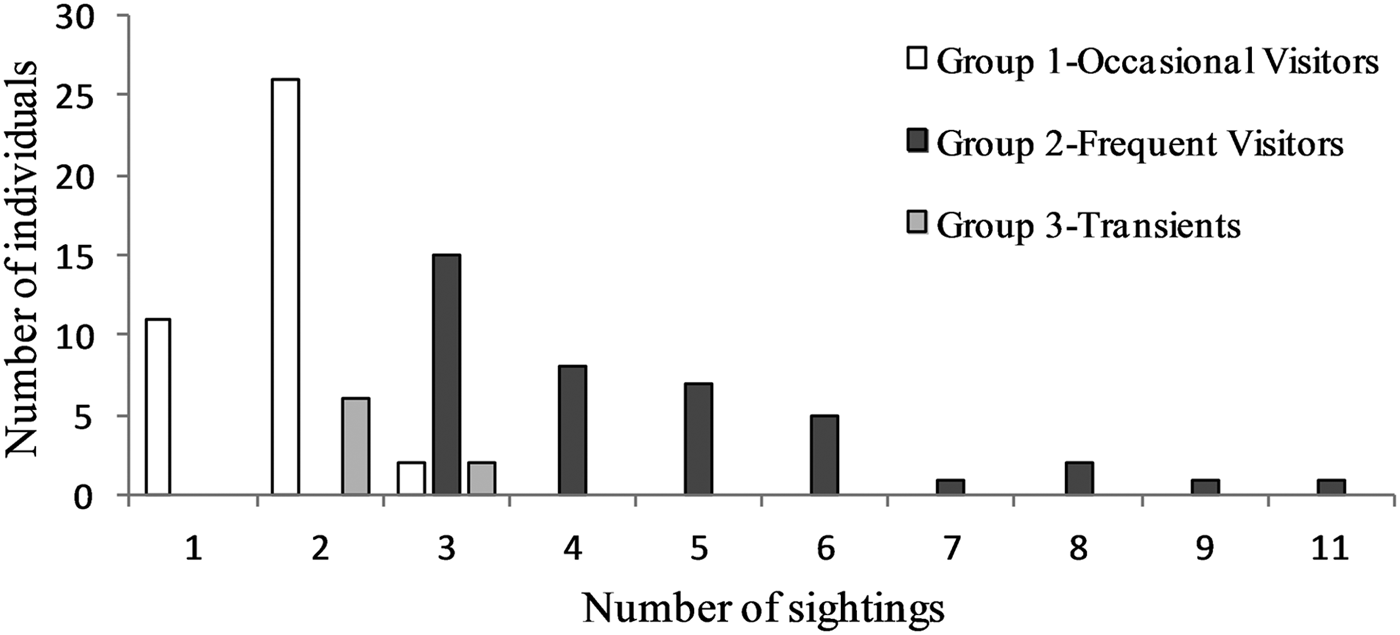
Fig. 3. Sighting frequency of bottlenose dolphins within the Istanbul Strait, according to the groupings of agglomerative hierarchical cluster analysis.
Table 4. Mean seasonal and monthly residency rates and site fidelities of bottlenose dolphins according to the groupings of the agglomerative hierarchical cluster analysis (SD, Standard deviation, Min, Minimum, Max, Maximum, ind., individual)
Discussion
Bottlenose dolphins have been the focus of various studies in the Istanbul Strait since the early 1900s (Deveciyan, Reference Deveciyan1926; Çelikkale et al., Reference Çelikkale, Ünsal, Durukanoğlu, Karaçam and Düzgüneş1988; Öztürk, Reference Öztürk1996; Öztürk & Öztürk, Reference Öztürk and Öztürk1996, Reference Öztürk, Öztürk, Evans, Parsons and Clark1997; Öztürk et al., Reference Öztürk, Dede and Tonay2009; Baş et al., Reference Baş, Öztürk and Öztürk2015; Bas et al., Reference Bas, Christiansen, Öztürk, Öztürk, Erdogan and Watson2017a, Reference Bas, Christiansen, Öztürk, Öztürk and Mcintosh2017b). Yet only a handful of studies have touched on the topic of habitat use (Dede, Reference Dede1999; Öztürk et al., Reference Öztürk, Dede and Tonay2009; Altuğ et al., Reference Altuğ, Altan, Oral, Topaloğlu, Dede, Kesin, İşinibilir, Çardak and Çiftçi2011; Ryan et al., Reference Ryan, Cucknell, Romagosa, Boisseau, Moscrop, Frantzis and McLanaghan2014). In the current study, we present the results of the first photo-identification study of bottlenose dolphins in the Istanbul Strait, reporting encounter rates, residency patterns and site fidelities. Based on our results, a relatively large number of bottlenose dolphins inhabit the Istanbul Strait and its adjacent waters. In addition, the species showed a regular presence between and within the years in this biological corridor between the Mediterranean and the Black Sea that is exposed to various anthropogenic impacts.
The average encounter rate for the entire study period was four groups (22 individuals) per 10 km, with the highest encounter rate being 11 groups (75 individuals) per 10 km in the south section during spring months. This contrasts with the previous findings from 2008, which reported 0.76 groups (0.143 sightings per nmile) per 10 km (Öztürk et al., Reference Öztürk, Dede and Tonay2009). A possible explanation for these considerably different encounter rates could be simply a variation in the survey methodology (including survey coverage and survey effort) or the actual fraction of the dolphin populations. This inconsistency in the results highlights that following similar survey protocols between years in the similar vicinity areas is crucial in being able to make spatio-temporal comparisons to inform management bodies. In the current situation, the difference in the encounter rates between the studies does not allow the formation of accurate explanations.
Nonetheless, the current study allows the comparison of encounter rates between seasons due to the similar temporal distribution of survey effort. Spring had the highest rate with five groups (34 individuals) per 10 km, while the rest of the seasons had three groups (18 individuals) per 10 km. Therefore, there was a similar rate of year-round presence of dolphins within the strait. However, encounter rates of dolphins showed temporal variations for each section. The adjacent waters of the Marmara Sea (south section) held the highest encounter rate for all seasons, with an average of nine groups (60 individuals) per 10 km. In contrast, the middle and middle-north sections held the lowest number of encounters for all seasons, with an average of one group (four individuals) per 10 km. The north part of the strait was used preferentially in spring and summer, with an average of six groups (37 individuals) and encounter rates dropped to one group (four individuals) in autumn and winter. To carefully interpret the uneven spatio-temporal distribution of dolphins, several factors need to be considered. It is suggested that dolphin distribution can be a result of a trade-off between predation risk and food availability (Heithaus & Dill, Reference Heithaus and Dill2002). With regard to food availability, the Istanbul Strait is the second most important fishing region in Turkey (TUIK, 2016) and hosts various pelagic fish species, such as the European anchovy (Engraulis encrasicolus), horse mackerel (Trachurus mediterraneus), sand smelt (Atherina sp.), European pilchard (Sardina pilchardus), red mullet (Mullus barbatus) and bonito (Sarda sarda) (Dede et al., Reference Dede, Öztürk, Akamatsu, Tonay and Öztürk2014). The named species show seasonal two-way migration; from Marmara to the Black Sea in spring and from the Black Sea to Marmara in autumn through the strait (Dede et al., Reference Dede, Öztürk, Akamatsu, Tonay and Öztürk2014). The same fish species were also reported in the diet of Black Sea bottlenose dolphins (Gladilina & Gol'din, Reference Gladilina and Gol'din2014). Therefore, the high encounter rate in spring at both the southern and northern entrances of strait is likely to be the result of high prey presence during the spring fish migration. This is supported by the results from Baş et al. (Reference Baş, Öztürk and Öztürk2015, Reference Bas, Christiansen, Öztürk, Öztürk, Erdogan and Watson2017a, Reference Bas, Christiansen, Öztürk, Öztürk and Mcintosh2017b) who found that travelling and diving (linked to foraging) were the dominant behavioural states within the total behavioural range of the dolphins in Istanbul Strait. However, the same behavioural pattern was only reported for the southern parts of the Strait during the autumn fish migration, while the rest of the strait showed almost no dolphin sightings, despite the high presence of fish in the area. The low number of dolphin sightings might be explained by high levels of human activities, ranging from marine transportation to fishing. The northern sections are legal fishing zones between September and April. Therefore, during autumn and the beginning of spring, fishing effort is intense in these sections of the strait, with over 50 purse seines reported at the same time and up to 100 purse seines reported daily in 5 km radius areas (Baş et al., Reference Baş, Öztürk and Öztürk2015). It is possible therefore, that the high density of purse seines and repetitive use of echo sounders during the fishing practices alter the bottlenose dolphin distribution from the northern sections in autumn and winter seasons, despite the high prey presence. Thus, it is possible that dolphins show area displacement during the fishing season and travel down to the adjacent waters of Marmara Sea to take the advantage of autumn fish migration. However, several studies have reported opportunistic feeding (begging) around fishing vessels, in contrast with the current results. Begging was mainly observed around trawlers (Genov et al., Reference Genov, Kotnjek, Lesjak, Hace and Fortuna2008; Hazelkorn et al., Reference Hazelkorn, Schulte and Cox2016; Kovacs et al., Reference Kovacs, Perrtree and Cox2017). In the Istanbul Strait, purse seines are the legal fishery practices, while trawling is illegal. Purse seines are one of the major threats to dolphin populations due to high entanglement and by-catch rates (Noren & Edwards, Reference Noren and Edwards2007; Marçalo et al., Reference Marçalo, Katara, Feijó, Araújo, Oliveira, Santos, Ferreira, Monteiro, Pierce, Silva and Vingada2015; Escalle et al., Reference Escalle, Pennino, Gaertner, Chavance, Delgado de Molina, Demarcq, Romanov and Merigot2016). It is also likely that the number of fishing vessels have an impact on the occurrence of this strategy. In the case of the Istanbul Strait, fishing vessel numbers can reach 100 in a day. Therefore, the number of fishing vessels in the vicinity of dolphins is likely to play a negative role in favouring this potential foraging strategy in the strait.
In addition to the heavy fishing pressure, the Istanbul Strait is also one of the busiest waterways in the world (Orakçı, Reference Orakçı, Oral and Öztürk2006; Birpınar et al., Reference Birpınar, Talu and Gönençgil2009) with over 45,000 vessels per year (Kara, Reference Kara2016). The middlemost sections had the highest density of marine traffic in the Istanbul Strait across all seasons (Baş et al., Reference Baş, Öztürk and Öztürk2015), with an average of 500 vessels a day (Table 1). The significant impact of vessels on the behavioural transitions of bottlenose dolphins in the Istanbul Strait have already been highlighted in a previous study (Bas et al., Reference Bas, Christiansen, Öztürk, Öztürk, Erdogan and Watson2017a, Reference Bas, Christiansen, Öztürk, Öztürk and Mcintosh2017b). Therefore, the annual low encounter rate of dolphins in the middle and middle-north sections could potentially be due to an avoidance behaviour linked to the heavy vessel traffic.
Based on the photo-identification data, at least 87 individuals were present in the study area between 2011 and 2013. Re-sighting data showed that at least 40 dolphins used the strait on a relatively regular basis between and within the 5 years. The reported number of individuals is possibly less than the actual total number of animals using the strait as poorly marked individuals or rarely sighted well-marked individuals also need to be taken into account. Many coastal bottlenose dolphin populations in the Mediterranean Sea are known to be highly resident, sometimes in semi-closed areas and relatively isolated from each other (Bearzi et al., Reference Bearzi, Notarbartolo di Sciara and Politi1997, Reference Bearzi, Politi and Natarbartolo di Sciara1999, Reference Bearzi, Agazzi, Bonizzoni, Costa and Azzellino2008; Fortuna, Reference Fortuna2007; Genov et al., Reference Genov, Wieman and Fortuna2009; Gnone et al., Reference Gnone, Bellingeri, Dhermain, Dupraz, Nuti, Bedocchi, Moulins, Rosso, Alessi, Mccrea, Azzellino, Airoldi, Portunato, Laran, David, Di-Méglio, Bonelli, Montesi, Trucchi, Fossa and Wurtz2011). The presence of frequent and occasional visitors in the strait suggests these could be part of a resident local population. However, the presence of transient dolphins also highlights that the Istanbul Strait might only be part of their distributional range. Indeed, identified individuals were re-sighted from the southern to the northern surrounding waters of the Istanbul Strait, with a maximum re-sighting distance of 35 km from their initial sighting.
Lastly, the current study took advantage of opportunistic data collected between 2014 and 2016. Even though the photographs were valuable to prove that certain individuals were indeed frequently visiting the strait between the years of 2011 and 2016, it also had drawbacks. The survey effort was by no means equal between 2011–2013 and 2014–2016, which in return resulted with low re-sightings between 2013 and 2016. This is likely to result in an underestimation of the residency and site fidelity indices. Continuous dedicated surveys are, therefore, recommended for future studies to improve the current understanding of the residency patterns.
To conclude, the current study showed that the Istanbul Strait together with its surrounding waters both in the Marmara and Black Sea, serves as an important area for bottlenose dolphins with a regular, within- and between-years presence. The Istanbul Strait is the only water connection path between the Black Sea and Mediterranean Sea, and is thus under heavy marine traffic and fishing pressures. Increasing human pressure, therefore, can have a detrimental effect not only for the Black Sea but also for the Mediterranean subpopulations.
Until species-level conservation and management actions can be tailored, marine traffic and fishing pressures should be minimized in the south and north section, with a need for extra measures in the middle and middle-north sections to allow the dolphins to move between the Black Sea and Mediterranean Sea. Continuous photo-identification surveys from different research institutes with inter-regional collaborations are of great importance to improve our understanding of the home range and population status of bottlenose dolphins that are currently listed as highly threatened in the Mediterranean and Black Sea. The knowledge gained from the current study, when combined with information from similar studies along the coasts of the Black Sea, Marmara Sea, Aegean Sea and Mediterranean Sea, can be vital to elaborate future management plans for cetaceans in this basin.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0025315418000577.
Acknowledgements
We would like to thank all the research assistants, fishermen and villagers throughout the process of collecting the data, Ilke Ertem for improving the manuscript and Kate Yeoman, Sarah Tubbs and Holly Waller for proofreading it. Moreover we would like to thank Istanbul University for their financial support. Finally, we are grateful to the editor of Journal of the Marine Biological Association of the United Kingdom, Dr Marie Louis and the two anonymous reviewers, for improving our manuscript immensely with their comments.