Introduction
Pagurus middendorffii Brandt, 1851 (Anomura: Paguridae) is a hermit crab inhabiting coastal waters in the Pacific Ocean from Olyutorsky Bay to the northern Honshu (Japan) and Korea, and from the Aleutian Islands to Vancouver (Canada) (Kim and Kwon, Reference Kim and Kwon1988; Nagasawa et al., Reference Nagasawa, Lützen and Kado1996; Marin, Reference Marin2013). This species is distributed up to 50 m depth, on sandy and silty-sandy bottoms, and may occur in the intertidal baths. In 1971–1975 and 1987, P. middendorffii was the dominant species in the whole Peter the Great Bay of the Sea of Japan (Volvenko, Reference Volvenko1995). In different parts of Vostok Bay (inner gulf of Peter the Great Bay), the proportion of this species varied from 33 to 100% (Pogrebov and Kashenko, Reference Pogrebov, Kashenko and Kasyanov1976) or was 45.6% (Volvenko, Reference Volvenko1995) of the total abundance of all coastal hermit crabs. However, in 2014–2015, its density decreased significantly to 1.3% of the total abundance of hermit crabs (Selin et al., Reference Selin, Kornienko and Korn2016).
Pagurus middendorffii is the host of parasitic isopods of the family Bopyridae including Athelges japonicus Shiino, Reference Shiino1958 (Shiino, Reference Shiino1958), A. takanoshimensis Ishii, 1914 (Kim and Kwon, Reference Kim and Kwon1988), Erimitione lata (Shiino, Reference Shiino1958) (Shiino, Reference Shiino1958), and also the colonial parasitic barnacle Peltogasterella gracilis (Boschma, 1927) (Rhizocephala: Peltogasterellidae) (Nagasawa et al., Reference Nagasawa, Lützen and Kado1996). In 2014–2015, 35.4% of P. middendorffii specimens from Vostok Bay were infested by isopods and rhizocephalans: 20.83% by P. gracilis (Figure 1A, B), 14.58% by A. takanoshimensis, and rarely by E. lata. Some hermit crabs carried P. gracilis + A. takanoshimensis or E. lata + A. takanoshimensis simultaneously (Kornienko et al., Reference Kornienko, Korn and Selin2018). It is possible that the high infestation of the P. middendorffii population and parasitic castration of the hosts was the reason for the large decline in abundance of this hermit crab in Peter the Great Bay (Selin et al., Reference Selin, Kornienko and Korn2016).

Figure 1. Host crab, Pagurus middendorffii, infested by Peltogasterella gracilis (A, B) and Peltogaster lineata (С, D).
Until now, the only rhizocephalans recorded on P. middendorffii was P. gracilis (McDermott et al., Reference McDermott, Williams and Boyko2010; Kornienko et al., Reference Kornienko, Korn and Selin2018). In 2021, we found a P. middendorffii specimen with a single (not colonial) rhizocephalan in Vostok Bay. This parasite was similar to Peltogaster lineata Shiino, Reference Shiino1943 recorded earlier on the hermit crab Pagurus brachiomastus (Thallwitz, 1891) in this locality (Golubinskaya et al., Reference Golubinskaya, Korn, Sharina and Miroliubov2021a). In 2023, two more hermit crabs with this parasite were found. In the present work, these rhizocephalans are identified using morphological and molecular methods.
Material and methods
Specimens of P. middendorffii infested by rhizocephalans (Figure 1C, D) were collected by SCUBA diving in Vostok Bay (42°53′N, 132°43′E) at a depth of 0.5–1 m, in September 2021 and 2023. These hermit crabs were deposited at the Museum of the A.V. Zhirmunsky National Scientific Center of Marine Biology, FEB RAS, Vladivostok (MIMB).
Material examined
One specimen (6.1/2.7 mm, with embryos without eyes), on P. middendorffii (male, 3.4 mm, depth 0.5 m, Vostok Bay, 5.09.2021) (catalogue number, MIMB 48412).
One specimen (5.0/1.8 mm, with embryos without eyes), on P. middendorffii (female, 4.1 mm, depth 1.0 m, Vostok Bay, 11.09.2023) (catalogue number, MIMB 48413); a part of the cuticle was used for SEM.
One specimen (6.3/2.0 mm, with embryos without eyes), on P. middendorffii (female, 4.0 mm, depth 1.0 m, Vostok Bay, 11.09.2023); was used for histology.
Molecular techniques
Two specimens of the rhizocephalan parasite and one specimen of P. middendorffii were fixed in 96% ethanol and subjected to molecular analysis. Total genomic DNA was extracted from the externae and from the tissue of crab chela using a chelating resin Chelex 100 (Bio-Rad, USA, CA) according to the protocol described by HwangBo et al. (Reference HwangBo, Son, Lee, Min, Ko, Liu, Choi and Jeong2010). The gene fragments of COI and 16S rDNA sequences were retrieved as in Golubinskaya et al.'s (Reference Golubinskaya, Korn, Sharina and Miroliubov2021a) study.
All sample PCR products were sequenced for both heavy and light strands, in order to improve accuracy, and aligned using MUSCLE (Edgar, Reference Edgar2004) implemented in MEGA v.11.0.8 (Tamura et al., Reference Tamura, Stecher and Kumar2021). Additional data for outgroup and ingroup taxa were taken from GenBank (NCBI, https://www.ncbi.nlm.nih.gov/). Phylogenetic trees were built for the genus Peltogaster (Figures 2 and 3) and for the genus Pagurus (Figures S1 and S2). Accession numbers for all taxa were included in alignments and phylogenetic analyses can be found in Table S2. The best-fit model of nucleotide substitution for the data sets was identified using ModelFinder (Kalyaanamoorthy et al., Reference Kalyaanamoorthy, Minh, Wong, von Haeseler and Jermiin2017) on the IQ-TREE webserver (http://www.iqtree.org/) (Trifinopoulos et al., Reference Trifinopoulos, Nguyen, von Haeseler and Minh2016). For the parasite specimens, TIM3 + F + I + G4 model was selected as the best for COI, and TPM3u + F + G4 model for 16S. For the host specimen, TVM + F + I + G4 model was chosen for COI and K3Pu + F + I + G4 model for 16S.
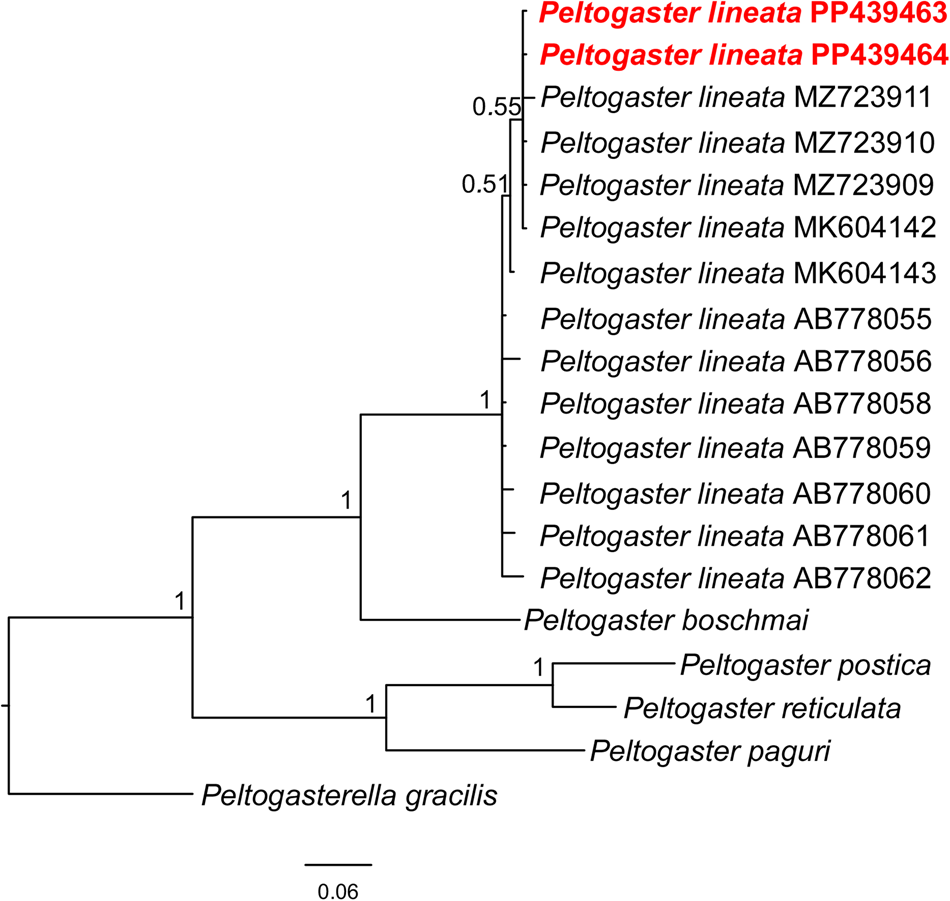
Figure 2. Bayesian phylogenetic tree for the COI dataset. The phylogeny shows the position of samples from this study within a monophyletic Peltogaster lineata. Nodal support is indicated in the form of Bayesian posterior probabilities (pp).

Figure 3. Bayesian phylogenetic tree for the 16S dataset. The phylogeny shows the position of samples from this study within a monophyletic Peltogaster lineata. Nodal support is indicated in the form of Bayesian posterior probabilities (pp).
For Bayesian analysis, we used the closest appropriate models. Bayesian trees were constructed using MrBayes 3.2.7a (Ronquist et al., Reference Ronquist, Teslenko, van der Mark, Ayres, Darling, Höhna, Larget, Liu, Suchard and Huelsenbeck2012) implemented in CIPRES Science Gateway (http://www.phylo.org/) (Miller et al., Reference Miller, Pfeiffer and Schwartz2010) with the following parameters: 10,000,000 generations, with four parallel chains and sample frequencies set to 500 in two separate runs. Based on the convergence of likelihood scores, 25% of the sampled trees were discarded as burn-in. All results trees were visualized using FigTree v. 1.4.4 (Rambaut, Reference Rambaut2012). The pairwise genetic distances were calculated using MEGA v.11.0.8 (Tamura et al., Reference Tamura, Stecher and Kumar2021). Intra- and interspecific nucleotide variability of the analysed species was based on the Kimura 2-parameter model (K2P) (Kimura, Reference Kimura1980) for COI sequences and uncorrected genetic distances were calculated for 16S sequences.
All resulting sequences were submitted to the National Center for Biotechnology Information (GenBank, NCBI, https://www.ncbi.nlm.nih.gov/) nucleotide database. The following are the accession numbers for P. lineata: COI, PP439463–PP439464, 16S, PP440164–PP440165 (Table 1 and Table S1). The accession numbers for P. middendorffii are: COI, PP439465, 16S, PP440166.
Table 1. Genbank accession details for Peltogster lineata used in phylogenetic analyses

Morphological investigation of the externa
An infested specimen of P. middendorffii was photographed alive. Microscopic examination of rhizocephalan was conducted under MBS-10 stereomicroscope and Olympus CX41 microscope. The following measurements of the parasite externae were made: length (distance between anterior and posterior ends) and width at the level of the stalk. The length of the anterior calcified part of the carapace (shield length) of the host hermit crab was also measured. Sex of the host was determined based on the presence of four unpaired pleopods in females and three unpaired pleopods in males (Tudge et al., Reference Tudge, Asakura, Ahyong, Schram and von Vaupel Klein2012). The location of the rhizocephalan externa on each host crab was noted.
One mature externa of the rhizocephalan was fixed in Bouin solution, dehydrated through a gradient ethanol-xylene series and embedded in paraffin. Transverse sections, 6 μm thick, were stained with Ehrlich haematoxylin (Kiernan, Reference Kiernan2015). Material was examined with a Zeiss Axio Imager Z.2 light microscope furnished with a digital camera. For SEM, the mantle of one externa was dehydrated through an ethanol series and acetone, critical point dried in CO2, and sputtered with chromium. It was observed with a Zeiss Sigma 300 VP microscope.
Results
Molecular analysis
For host, the length sequences were 543 bp for COI and 458 bp for 16S and after alignment and trimming to the same length the final data set was 479 and 334 bp, respectively. All sequences belonging to P. middendorffii form the same clade with low value of genetic distances between them. For COI, genetic distance between our (PP439465) and other sequences was 1.27 ± 0.51% (mean ± standard error), and intraspecific distance was 0.42 ± 0.3%. Here and further, intraspecific genetic distances were calculated without including own data. For 16S, these values were 0.04 ± 0.04% between our (PP439466) and other sequences with intraspecific distance 0.09 ± 0.08%. Thus, the identity of the hosts sample as P. middendorffii was confirmed by molecular analysis (Figures S1 and S2).
For parasite, the sequence length for our data was 574 ± 674 bp for COI and 449 ± 464 bp for 16S. However, sequences from GenBank (NCBI) were included in current analysis that contains incompletely overlapping fragments because of using the different primer pairs for analysed gene fragments. The final data set after alignment and trimming to the same length was 385 and 263 bp, respectively. Phylogenetic trees for both COI and 16S rDNA showed that sequences retrieved for this research form a single monophyletic clade with other sequences of P. lineata (which were obtained from different research and from different crab hosts) (Figures 2 and 3, Table 1 and Table S1). Genetic distances between our data and the data obtained from GenBank (NCBI) were 0.83 ± 0.29% for COI and 0.44 ± 0.2% for 16S with the following value of intraspecific distances 1.03 ± 0.28% and 0.43 ± 0.21% for COI and 16S, respectively. Thus, the comparison of pairwise genetic distances also indicated the absence of significant differences between analysed sequences of P. lineata for both fragments (Tables S2 and S3). So, the molecular analysis with the use of the markers COI and 16S showed that our rhizocephalans found parasitizing the hermit crab P. middendorffii belong to P. lineata.
Taxonomy
Superorder Rhizocephala Müller, 1862
Family Peltogastridae Lilljeborg, 1861; amended by Høeg et al. (Reference Høeg, Noever, Rees, Crandall and Glenner2020)
Genus Peltogaster Rathke, 1842
Peltogaster lineata Shiino, Reference Shiino1943
Peltogaster lineatus – Shiino, Reference Shiino1943:23–24, fig. 16.
Peltogaster lineata – Yoshida et al., Reference Yoshida, Hirose and Hirose2014: 471, 473, figs. 2C–E, 3B; Jung et al., Reference Jung, Yoshida and Kim2019: 7–8, figs. 2A, 5A; Golubinskaya et al., Reference Golubinskaya, Korn, Sharina and Miroliubov2021a.
Short description of the externa
We found one male and two females of P. middendorffii each bearing single externa of P. lineata. Each externa was attached near the second pleopod of the host. The shield length of infested hermit crabs ranged from 3.4 to 4.1 mm. The mature externae with embryos without eyes were pale, oval and slightly curved. The size of the externae ranged from 5.0 to 6.3 mm in length and from 1.8 to 2.7 mm in width. The anterior end was broad and bilobed, with the mantle opening placed subterminally on the side facing of the host. The mantle opening was U-shaped, slightly elevated and inserted between the two slightly projecting subequal lobes. The conspicuous fusiform shield covered near 1/4 of the externa. A very short stalk was located on the midpoint of the body axis.
A visceral sac with large developing oocytes extended dorsally along most of the externa (Figure 4A). The mantle cavity was closely filled with embryos with a diameter from 110 to 130 μm. The mantle was ca. 50 μm in thickness (Figure 4B). The colleteric glands were short strongly flattened tubes with a size ranging from 293 × 37 to 443 × 72 μm, placed within the shield level (Figure 4A, C). They gradually passed into thin posterior parts, with a diameter 67–79 μm. Short tubular receptacles, with a diameter of 65–118 μm, were placed inside the visceral sac within the shield level (Figure 4D). The receptacles gradually passed into receptacle ducts with a diameter of 31–63 μm (Figure 4E). The posterior (distal) parts of the receptacle ducts were coiled and opened on the lateral surfaces of the visceral mass.

Figure 4. Histology of the externa Peltogaster lineata. Transverse section of the whole externa (A); mantle (B); strongly flattened colleteric gland (C); receptacles (D); coiled receptacle duct (E). cg, colleteric gland; em, embryon; m, mantle; mc, mantle cavity; ov, ovary; rd, receptacle duct; re, receptacles.
The external cuticle was smooth, without papillae or excrescences (Figure 5A). The mantle opening was surrounded by lips densely covered with numerous spines (Figure 5B, C). The internal cuticle was wrinkled and covered with sparse papillae (‘hairs’) (Figure 5D). Numerous clavate barbed retinacula of 12–14 μm in length occurred singly, some of them were placed in depressions (Figure 5E, F).

Figure 5. SEM showing cuticle structure of the externa of Peltogaster lineata. Smooth external cuticle (A); mantle opening (B); numerous spines densely covering mantle opening (C); wrinkled internal cuticle covered with papillae (‘hairs’) (D); clavate barbed retinaculum (E, F).
Discussion
The morphological investigation showed the great similarity between rhizocephalans parasitizing P. brachiomastus and P. middendorffii. They are similar in external shape, coloration and position on the host. Their cuticles are also nearly identical. At the same time, these species differ in two anatomical characters. The mantle of the parasite from P. middendorffii is considerably thinner than that from P. brachiomastus (50 vs 100 μm). Moreover, its colleteric glands are strongly flattened and reach greater diameter (443 vs 239 μm) (Golubinskaya et al., Reference Golubinskaya, Korn, Sharina and Miroliubov2021a). These differences may be due to the large number of developing eggs in the mantle cavity, which could cause the stretching of the mantle and flattening of the oviducal glands. The molecular analysis herein confirmed that the rhizocephalans found on P. middendorffii as well as on hermit crabs Pagurus filholi (de Man, 1887), P. nigrivittatus Komai, 2003 and P. maculosus Komai & Imafuku, 1996 were all P. lineata.
In rhizocephalans, multi-species infestation of a single host is not rare; however, previously, it was believed that different parasites are rarely found sympatrically. In 2006, three species, Sacculina confragosa Boschma, 1933, S. imberbis Shiino, 1843 and Parasacculina yatsui (Boschma, 1936), were found parasitizing a single host crab, Pachygrapsus crassipes Randall, 1840, in a restricted locality (Tsuchida et al., Reference Tsuchida, Lützen and Nishida2006). Last years, we observed two examples of parasitism with two sympatric rhizocephalans on the same host in Peter the Great Bay. Two congeneric species, Lernaeodiscus rybakovi Korn, Golubinskaya, Rees, Glenner & Høeg, Reference Korn, Golubinskaya, Rees, Glenner and Høeg2020 and L. kasyanovi Korn, Golubinskaya, Rees, Glenner & Høeg, Reference Korn, Golubinskaya, Rees, Glenner and Høeg2021, infest the anomuran crab Pachycheles stevensii Stimpson, 1858 (Korn et al., Reference Korn, Golubinskaya, Rees, Glenner and Høeg2020, Reference Korn, Golubinskaya, Rees, Glenner and Høeg2021). The parasitization of the spider crab Pugettia aff. ferox Ohtsuchi & Kawamura by two sacculinids, Sacculina pugettiae Shiino, Reference Shiino1943 and Parasacculina pilosella (Van Kampen et Boschma, 1925), from different genera and families, is an example not only of sympatric multi-species infestation, but the first finding of two different parasites on a single crab specimen (Golubinskaya et al., Reference Golubinskaya, Korn, Sharina and Selin2021b). Pagurus middendorffii is another crab infested by two parasites, Peltogasterella gracilis and P. lineata, from different rhizocephalan families in the same locality.
Peltogaster lineata parasitizes numerous crab species. In Seto, this species occurs on Pagurus japonicus (Shiino, Reference Shiino1943). Yoshida et al. (Reference Yoshida, Hirose and Hirose2014) did not find P. japonicus infested by P. lineata along the Pacific coast of Honshu, but revealed three new hosts for this rhizocephalan: P. filholi, P. nigrivittatus and P. maculosus. In Japan, the main host of P. lineata was P. nigrivittatus; its prevalence reached 70%, followed by P. filholi at 20%, and P. maculosus as only 10% of the examined material. The fifth host of P. lineata, P. brachiomastus, was found in Korea; the prevalence of P. filholi and P. brachiomastus was 50% each (Jung et al., Reference Jung, Yoshida and Kim2019). In Russian waters, P. lineata was so far found only on P. brachiomastus (Golubinskaya et al., Reference Golubinskaya, Korn, Sharina and Miroliubov2021a). Pagurus middendorffii is the sixth known host of P. lineata. The host preference of P. lineata changes from P. filholi + P. nigrivittatus + P. maculosus in Japan to P. filholi + P. brachiomastus in Korea (Yoshida et al., Reference Yoshida, Hirose and Hirose2014; Jung et al., Reference Jung, Yoshida and Kim2019), and to P. brachiomastus + P. middendorffii in Russia.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0025315424000511
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on request.
Author contributions
N. A. A. collected the material. O. M. K. and D. D. G. studied morphological characters. O. M. K. wrote the manuscript. D. D. G. performed all illustrations. S. N. S. performed the molecular analysis. All authors read and approved the manuscript.
Financial support
The study was funded by the Federal scientific and technical programme in the field of environmental development of the Russian Federation and climate change for 2021–2030, Russian Federation (project no. 123080800009-5).
Competing interest
None.
Ethical standards
All applicable international, national and/or institutional guidelines for the care and use of animals were followed by the authors.








