Introduction
Slipper lobsters of the family Scyllaridae are widespread in shallow, warm temperate and tropical habitats. Their life cycle includes several planktonic phyllosoma stages, lasting weeks to months, and a final phyllosoma instar that metamorphoses into nisto, a nektonic postlarva that transforms to the benthic adult (Lavalli and Spanier, Reference Lavalli and Spanier2007). Among scyllarids, the pygmy slipper lobster, Biarctus sordidus (Stimpson, Reference Stimpson1860), is one of the smallest species. Its distribution spans across the Indian Ocean, from Australia, Indonesia, Borneo, Philippines, Singapore, Malaysia, Thailand, South China and the Persian Gulf, at shallow depths of 2.7 to 73 m (Holthuis, Reference Holthuis2002). Phyllosoma larvae of B. sordidus were reported from Australia (Barnett, Reference Barnett1989; Mcwilliam et al., Reference Mcwilliam, Phillips and Kelly1995), Japan (Sekiguchi, Reference Sekiguchi1997), Java (Tampi and George, Reference Tampi and George1975), and India (George and Thomas, Reference George and Thomas1997; Sankolli and Shenoy, Reference Sankolli and Shenoy1973). However, neither adults nor larvae of B. sordidus were previously recorded in the Red Sea.
Identifying scyllarid phyllosomata to species based on their morphology is difficult due to incomplete descriptions or assignments of developmental stages, as well as disagreements among authors regarding the distinguishing characteristics of these stages (Pagliarino et al., Reference Pagliarino, Massi, Canali, Costa, Pessani and Bianchini2013). Employing integrative taxonomy by combining DNA barcoding and morphological characterization is therefore essential for establishing the phyllosoma larval stages of scyllarid species (Guy-Haim et al., Reference Guy-Haim, Iakovleva, Ermak, Spanier and Morov2024). Here we used integrative taxonomy to describe an early stage phyllosoma of B. sordidus, recorded for the first time in the Red Sea.
Materials and methods
A plankton sample was collected in 6 March 2024 at 6:00 AM using a plankton net (200-μm mesh size, 50 cm opening diameter, Aquatic Research Instruments, USA), towed horizontally for 10 min at a depth of 0.5–1 m in Station A, located at the northern Gulf of Aqaba, Eilat, bottom depth ca. 700 m (29°28′N, 34°55′E, Figure 1A). Fieldwork was conducted under INPA permit no. 2024-43522.
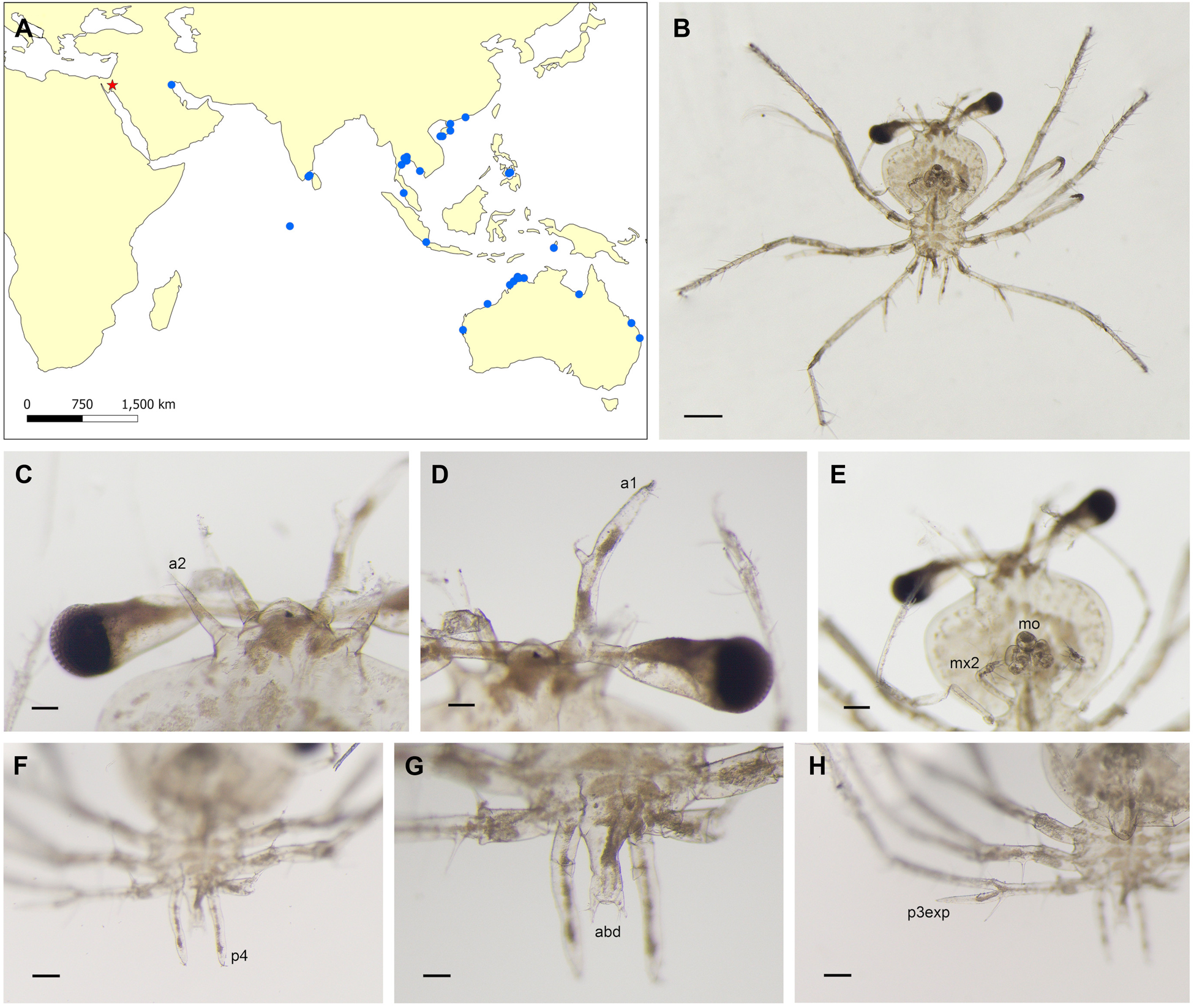
Figure 1. A. The distribution map of Biarctus sordidus. Occurrences are presented in blue circles and the new record in a red star. Occurrences were downloaded from https://www.gbif.org/ and OBIS https://obis.org/ on 17 April 2024. Additional occurrences were added from Holthuis (Reference Holthuis2002). B–H. B. sordidus phyllosoma stage III/IV. B. ventral view. C. Antenna (a2). D. Antennule (a1). E. Mouth (mo) and maxillipeds. F. Pereiopod 4 (p4). G. Abdomen (abd). H. Exopod of pereiopod 3 (p3exp). Scale bars: B – 500 μm, C – 100 μm, D – 100 μm, E – 500 μm, F – 200 μm, G – 100 μm, H – 100 μm.
The plankton sample was examined under a stereomicroscope (SZX16, Olympus, Japan). A phyllosoma larva was identified morphologically and the developmental stage was determined following Sankolli and Shenoy (Reference Sankolli and Shenoy1973) and Ritz (Reference Ritz1977). One pereiopod was cut for molecular identification, and the specimen was stored in 70% ethanol and deposited in the zooplankton collection, the National Natural History Collections, Hebrew University of Jerusalem (NNHC, HUJI).
Total genomic DNA was extracted from the pereiopod using the InviSorb Spin Tissue Mini Kit (Invitek Diagnostics, Germany) according to the manufacturer's specifications. Following the DNA extraction, the 18S rRNA gene was amplified using the primers 18S-3F and 18S-9R following Giribet et al. (Reference Giribet, Carranza, Riutort, Baguñà and Ribera1999) and the mitochondrial 16S gene was amplified using the primers 16Sar-16Sbr following Palumbi (Reference Palumbi1996). Reaction conditions were as follows: 94°C for 2 min, followed by 34 cycles of 94°C for 15 s, 49°C for 30 s, and 72°C for 1 min, and a final elongation step of 72°C for 7 min. Obtained PCR products were purified and sequenced by Hylabs (Rehovot, Israel).
A total of twenty-one 18S rRNA sequences of Scyllaridae were analysed, including one sequence of the phyllosoma of B. sordidus obtained in this study, one sequence of adult B. sordidus from Thailand (JN701622), one sequence of B. vitiensis from Guam (JN701621), and additional 18 scyllarid sequences obtained from GenBank (accession numbers and collection locality are indicated in Figure 2). Ibacus peronii and I. chacei were used as an outgroup.
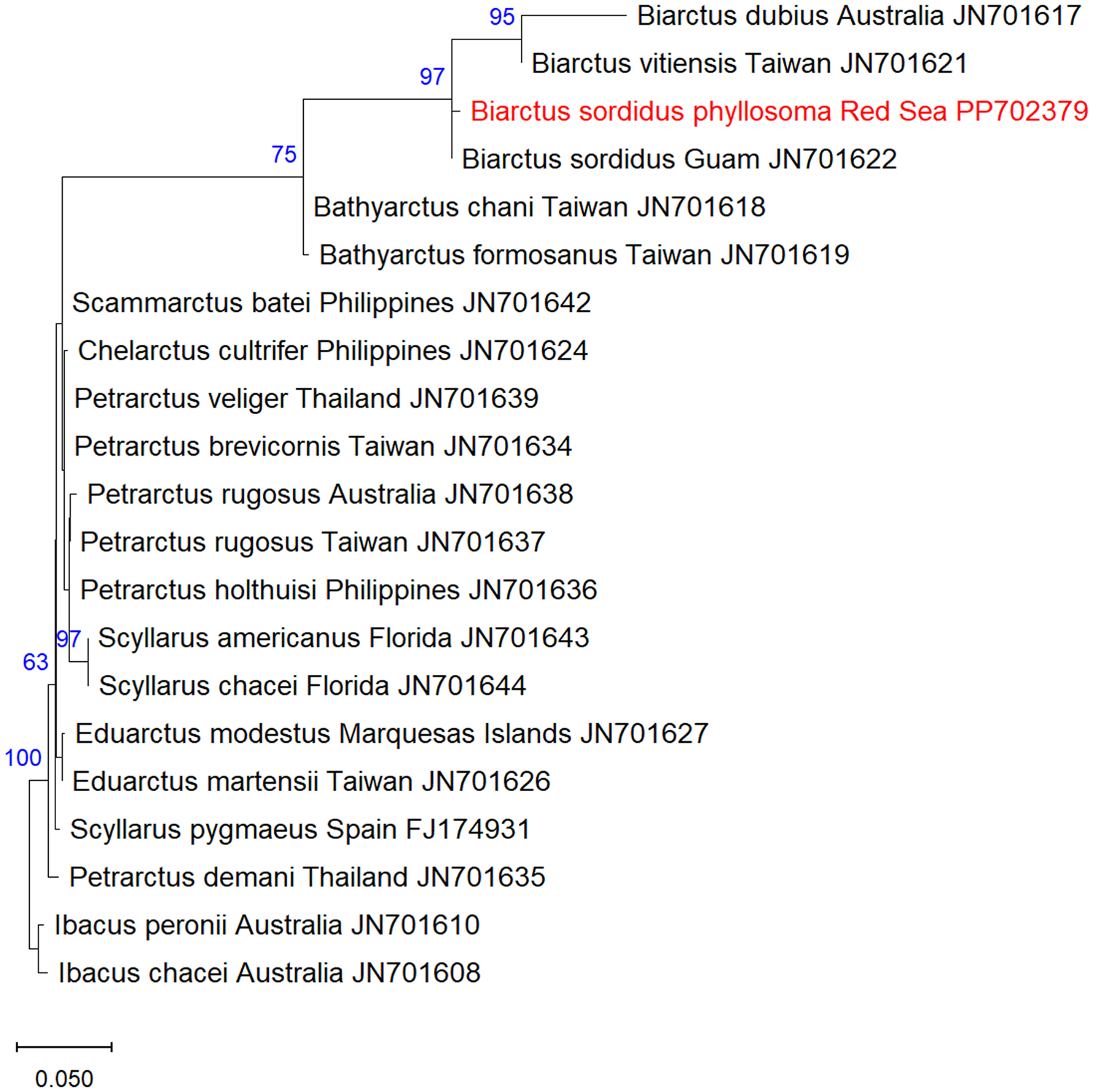
Figure 2. Maximum-Likelihood phylogenetic tree of Biarctus sordidus based on the 18S rRNA gene, using the K2 + G substitution model. The outgroups Ibacus peronii and I. chacei were used as a root node. The numbers in blue indicate the percentage of ML bootstrap support (1000 replicates) for nodes that received at least 60% support. The scale bar denotes the estimated number of nucleotide substitutions per site.
A total of fifteen 16S mtDNA sequences of Scyllaridae were analysed, including one sequence of the phyllosoma of B. sordidus obtained in this study, two sequences of adult B. sordidus from Thailand (JN701710) and Australia (AY583888), two sequences of B. pumilus from South Africa (ON960177- ON960178), one sequence of B. vitiensis from Guam (JN701709), and additional nine scyllarid sequences obtained from GenBank (accession numbers and collection locality are indicated in Figure 3). Ibacus chacei was used as an outgroup.
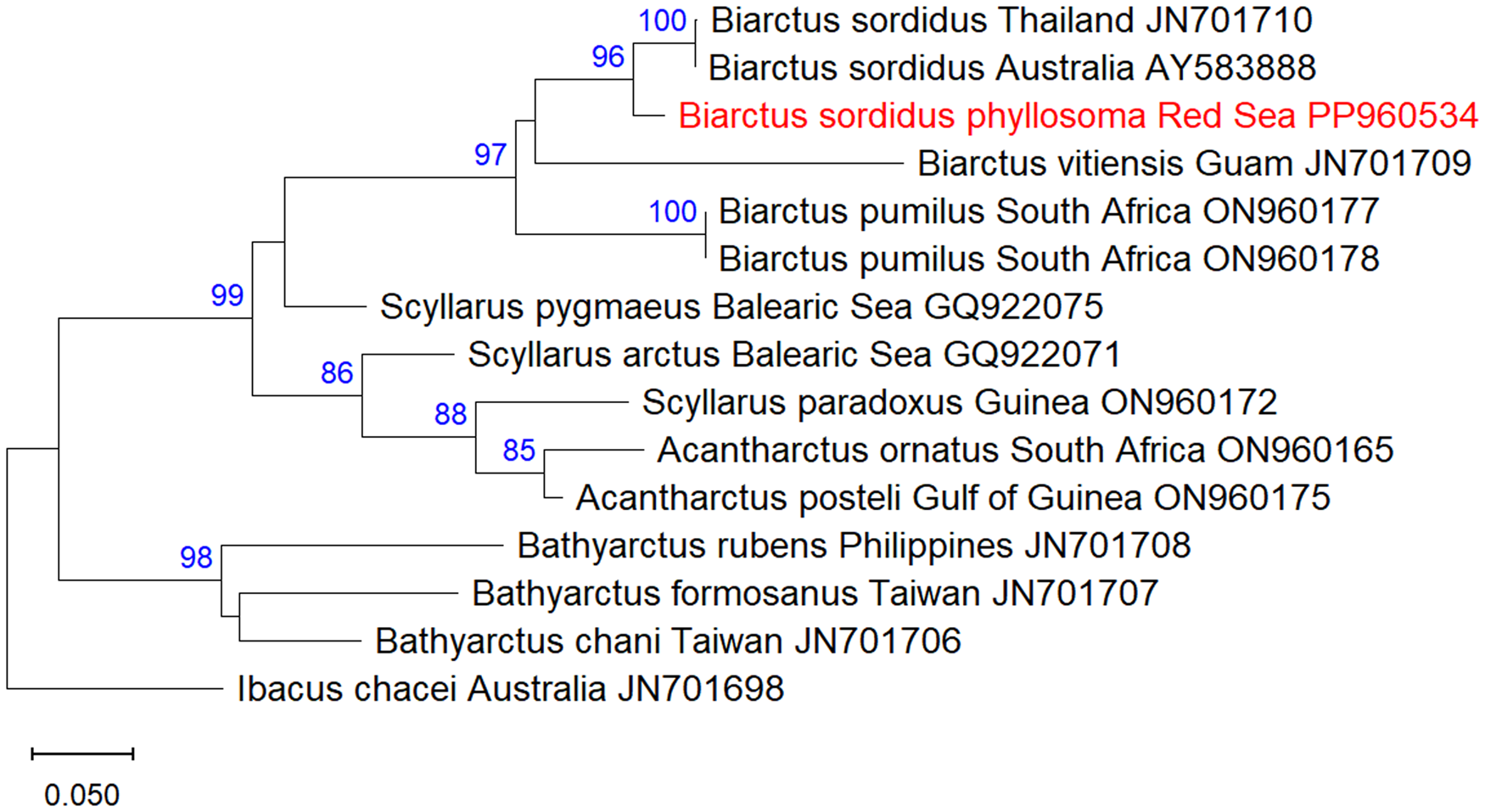
Figure 3. Maximum-Likelihood phylogenetic tree of Biarctus sordidus based on the mitochondrial 16S gene, using the HKY + G substitution model. The outgroup Ibacus chacei was used as a root node. The numbers in blue indicate the percentage of ML bootstrap support (1000 replicates) for nodes that received at least 60% support. The scale bar denotes the estimated number of nucleotide substitutions per site.
Sequence alignments were conducted using ClustalW embedded in MEGA v11.0 (Tamura et al., Reference Tamura, Stecher and Kumar2021). The best-fitting substitution models were selected according to the Bayesian Information Criterion using Maximum-likelihood (ML) model selection in MEGA. ML analyses were performed using the K2 + G (18S) and HKY + G (16S) models with 1000 bootstrapping replicates each.
Results
Morphological description
Biarctus sordidus (Stimpson, Reference Stimpson1860): phyllosoma, stage III (following Sankolli and Shenoy, Reference Sankolli and Shenoy1973) / IV (following Ritz, Reference Ritz1977) (Figure 1B).
Dimensions: TL (total length) 2.24 mm; CL (cephalic length) 1.52 mm; CW (cephalic width) 1.49 mm; ThW (thorax width) 0.73 mm; ThL (thorax length) 0.76 mm; A1L (antennular length) 0.63 mm; A2L (antennal length) 0.22 mm; AbdL (abdomen length) 0.21 mm.
Cephalic shield subcircular, twice wider than thorax. Eyestalks segmented. Antennule unsegmented with inner flagellum as a small bud. Right antennule damaged. Antenna short uniramous with small seta on either side of tip. Pereiopod 3 with unsegmented setose exopod. Pereiopod 4 more than twice longer than abdomen. Uropods not visible.
Molecular identification
A DNA fragment of 577 bp of the 18S rRNA gene was sequenced from the phyllosoma larva of B. sordidus and assembled from forward and reverse sequences. The sequences were deposited in NCBI GenBank under the accession numbers PP702379 (18S) and PP960534 (16S). NCBI blastn yielded 99.61% identity of the 18S sequence to B. sordidus from Guam, and 96.33% identity of the 16S sequence to B. sordidus from Thailand. Maximum likelihood analysis of 18S Scyllaridae sequences obtained from GenBank showed that the phyllosoma from the Red Sea is clustered together with B. sordidus within the Biarctus clade, with high bootstrap support.
Discussion
The Red Sea is home to seven scyllarid species: the clamkiller slipper lobster, Scyllarides tridacnophaga, the Aesop slipper lobster, S. haanii, the dark-spot locust lobster, Gibbularctus gibberosus, the flathead lobster, Thenus orientalis, the hunchback locust lobster Petrarctus rugosus, Eduarctus lewinsohni, and Biarctus pumilus (Holthuis, Reference Holthuis1968; Holthuis, Reference Holthuis2002). Here we report an eighth scyllarid in the Red Sea, Biarctus sordidus, following the finding of its phyllosoma larva in the northern tip of the Gulf of Aqaba, Eilat.
Using laboratory-hatched eggs of adults collected from Moreton Bay, southeast Queensland, Australia, Ritz (Reference Ritz1977) described the first phyllosoma stage of B. sordidus, and based on plankton samples he provided keys to its eight phyllosoma stages. Sankolli and Shenoy (Reference Sankolli and Shenoy1973) described the first six phyllosoma stages of laboratory hatched B. sordidus collected from western India, stating that ‘each phyllosoma stage (instar) took, on an average, five days to moult to the next’. Thus, it can be estimated that the larval duration of B. sordidus is approximately 40 days. We found the stage III/IV phyllosoma of B. sordidus in the beginning of March in the offshore waters of the northern Gulf of Aqaba. We can therefore hypothesize that a potential recruitment of this species in the Red Sea may take place during April.
Similar to its congener B. pumilus, B. sordidus inhabits shallow hard sandy bottoms within its distributional range. The location and shape of the cardiac and gastric teeth, minute morphological features, differentiate between these sister taxa (Holthuis, Reference Holthuis2002). Thus, it is plausible that former biodiversity surveys in the Red Sea have confused B. sordidus with B. pumilus. Another reason for the late detection of B. sordidus in the Red Sea might be a recent introduction by ship ballast, as was previously hypothesized to explain the introduction of the Caribbean spiny lobster Panulirus argus to Cape Verde Archipelago (Freitas and Castro, Reference Freitas and Castro2005). Cargo ships that arrived at the Port of Eilat in Israel and the Port of Aqaba in Jordan, both located at the Northern Gulf of Aqaba, could have been the vector of such an introduction. Nonetheless, there is no former evidence for ballast-mediated introductions of phyllosoma larvae. Further studies are needed to unveil whether the pygmy slipper lobster has established populations in the Red Sea.
Acknowledgements
We wish to thank Dr Miguel Frada, Yoav Avrahami, Gal Vered, Asa Oren, Maya Van Gelder, the R/V Sam Rothberg crew, and the students who attended the 2024 Introduction to Plankton course at the Interuniversity Institute for Marine Sciences, Eilat for their assistance in sampling.
Author contributions
T.G-H. has conceived the study, analyzed the data, interpreted the findings and written the article. A.I. and A.R.M. have analyzed the data. V.F. has collected the data. E.S. has assisted in the interpretation of the findings. All coauthors contributed to the writing of the article.
Competing interests
The authors declare no competing interests.
Data Availability Statement
The data underlying this article are available in the GenBank Nucleotide Database at https://www.ncbi.nlm.nih.gov/genbank/, and can be accessed with accession numbers PP702379.1 and PP960534.1.





