INTRODUCTION
Glass sponges (Class Hexactinellida) constitute a predominantly deep-sea group, typically occurring at bathyal and abyssal depths (Leys et al., Reference Leys, Mackie and Reiswig2007), encompassing over 620 species worldwide (Van Soest et al., Reference Van Soest, Boury-Esnault, Hooper, Rützler, de Voogd, Alvarez de Glasby, Hajdu, Pisera, Manconi, Schoenberg, Janussen, Tabachnick, Klautau, Picton, Kelly, Vacelet, Dohrmann, Díaz and Cárdenas2015). Although only 45 hexactinellid species are known to occur in the North-east Atlantic (Van Soest et al., Reference Van Soest, Boury-Esnault, Hooper, Rützler, de Voogd, Alvarez de Glasby, Hajdu, Pisera, Manconi, Schoenberg, Janussen, Tabachnick, Klautau, Picton, Kelly, Vacelet, Dohrmann, Díaz and Cárdenas2015) some species are reported to form dense monospecific populations in some areas, e.g. Pheronema carpenteri (Thomson, Reference Thomson1869) on the Porcupine Seabight (Rice et al., Reference Rice, Thurston and New1990), on the Azores islands (Topsent, Reference Topsent1892) and the continental slope off Morocco (Barthel et al., Reference Barthel, Tendal and Thiel1996); Nodastrella asconemaoida Dohrmann, Göcke, Reed & Janussen Reference Dohrmann, Göcke, Reed and Janussen2012 on the bathyal coral reefs of Rockall Bank (Van Soest et al., Reference Van Soest, Van Duyl, Maier, Lavaleye, Beglinger, Tabachnick, Custódio, Lôbo-Hajdu, Hajdu and Muricy2007 as Rossella nodastrella). Other hexactinellids are also common in the multispecific astrophorid grounds known as Ostur that occur in the boreo-Arctic region. Examples of such species are Asconema foliata (Fristedt, Reference Fristedt1887) found in the Flemish Cap and Grand Banks (Murillo et al., Reference Murillo, Durán Muñoz, Cristobo, Ríos, González, Kenchington and Serrano2012) and Schaudinnia rosea (Fristedt, Reference Fristedt1887) on the Denmark Strait (Klitgaard & Tendal, Reference Klitgaard and Tendal2004). Various key ecological roles, e.g. substrate consolidation, bentho-pelagic coupling, and as hiding- and brooding places for associated fauna have been attributed to sponges in general (see a review in Bell, Reference Bell2008). Apart from these, hexactinellid sponges in particular seem to further structure the deep-sea sediment, through spicule mats and stalks left upon their death, promoting the colonization by other invertebrates of this otherwise less suitable habitat, thus creating habitat islands (Bett & Rice, Reference Bett and Rice1992; Beaulieu, Reference Beaulieu2001). On account of their slow growth, high longevity and unknown reproductive and distribution patterns, deep-sea sponge aggregations are considered particularly vulnerable to anthropogenic activities such as bottom-fisheries, gas and oil exploration. For these reasons they are classified as vulnerable marine ecosystems of utmost conservation priority as expressed by the European Union Council Regulation no. 734/2008 and have been listed under the OSPAR convention list of threatened and/or declining species and habitats (OSPAR commission, 2008). Poliopogon amadou Thomson, Reference Thomson1878 is a large pheronematid originally described from a specimen collected during the HMS Challenger expedition, at a depth of 2790 m south-west of the Canary islands. Some other records of the species have followed (Tabachnick & Menshenina, Reference Tabachnick, Menshenina, Hooper and Van Soest2002) but none indicating that this species formed dense aggregations. Here we report such an aggregation observed at ~2700 m depth on the Great Meteor seamount.
MATERIALS AND METHODS
The seamounts south of the Azores were surveyed during a cruise organized in the scope of the Portuguese Continental Shelf Extension Programme (EMEPC/Açores/G3/2009 – EMEPC-Portugal) on board the NRP ‘Almirante Gago Coutinho’ (Figure 1A). The Great Meteor seamount, located at 29°30′N 28°17′W, is one of the largest seamounts of the North-east Atlantic. It steeply rises from more than 4500 m to less than 300 m and possesses a flattened summit of approximately 50 km in diameter (Figure 1B).

Fig. 1. Study area: (A) the seamounts located southern of the Azores archipelago; (B) the ROV ‘Luso’ (EMEPC-Portugal); (C) topographic contour of the Great Meteor seamount.
Samples were collected by the ROV ‘Luso’ (Figures 1C & 2A–C), sorted aboard and preserved in 96% ethanol until further processing. Identifications were made from thick tissue sections mounted in Canada balsam. Spicules were dissociated by nitric acid boiling, washed, and examined at high magnification under scanning electron microscopy (SEM).

Fig. 2. In situ observations and sampling of P. amadou: (A) Large aggregation at 2700 m on the Great Meteor seamount; (B, C) sampling of specimens.
SYSTEMATICS
Class HEXACTINELLIDA Schmidt, Reference Schmidt1870
Subclass AMPHIDISCOPHORA Schulze, Reference Schulze1886
Order AMPHIDISCOSIDA Schrammen, Reference Schrammen1924
Family PHERONEMATIDAE Gray, Reference Gray1870
Genus Poliopogon Thomson, Reference Thomson1878
DIAGNOSIS
Pheronematidae with a fan-like body in which the concave side represents the atrial cavity. Basalia are in relatively broad tufts and include some monaxones with clavate distal ends and two-toothed anchors. Choanosomal, hypodermal and hypoatrial spicules are pentactines, rarely stauractines and tauactines. Uncinates usually consist of only one type. Dermalia and atrialia are pinular pentactines and rare hexactines. Microscleres are amphidiscs (from one to three kinds) and combinations of microhexactines and pentactines (in some species also stauractines, diactines, monactines and spheres) (Tabachnick & Menshenina, Reference Tabachnick, Menshenina, Hooper and Van Soest2002).
Poliopogon amadou Thomson, Reference Thomson1878
EXAMINED MATERIAL
L09D12B03 (EMEPC/LUSO/2009 Expedition, Dive 12, Great Meteor seamount; coordinates: 29°30.5097′N 28°17.2138′W; water depth: 2765 m); coll. J.R. Xavier, 20 September 2009; L09D12B05(S2) (EMEPC/LUSO/2009 Expedition, Dive 12, Great Meteor seamount; coordinates: 29°30.4442′N 28°17.2385′W; water depth: 2703 m); coll. J.R. Xavier, 20 September 2009; L09D12B08 (EMEPC/LUSO/2009 Expedition, Dive 12, Great Meteor seamount; coordinates: 29°30.5090′N 28°17.2782′W; water depth: 2675 m); coll. J.R. Xavier, 20 September 2009; L09D13B01(S1) (EMEPC/LUSO/2009 Expedition, Dive 13, Closs seamount; coordinates: 29°19.2800′N 29°07.8680′W; water depth: 2761 m); coll. J.R. Xavier, 21 September 2009.
EXTERNAL MORPHOLOGY
White to yellow thick-walled involute plate, fan or tongue shaped (Figure 3A, B). The largest specimen is fan shaped, measures 35 cm in length and 27 cm in width (Figure 3B). The thickness of the plate varies between 5–7 cm at its centre and becomes gradually thinner towards the margin. The atrial (concave) and dermal (convex) surfaces are covered by a fine quadrangular network of spicules (Figure 3C). A wide basal tuft of large spicules measuring approximately 9 cm in length enables its attachment to the rocky substrate (Figure 3D). Upon preservation, specimens tinged the ethanol a dark fuchsia to dark purple and the sponge tissue turned purplish grey, as previously observed for this species by Moseley (Reference Moseley1877).

Fig. 3. Poliopogon amadou collected specimens: (A) dermal view of the tongue-shaped specimen collected on the Closs seamount; (B) atrial view of the large fan shaped specimen collected on the Great Meteor seamount; (C) detail of the atrial surface with its characteristic spicule network; (D) detail of the basal tissue-devoid tuft with long spicules.
SKELETON
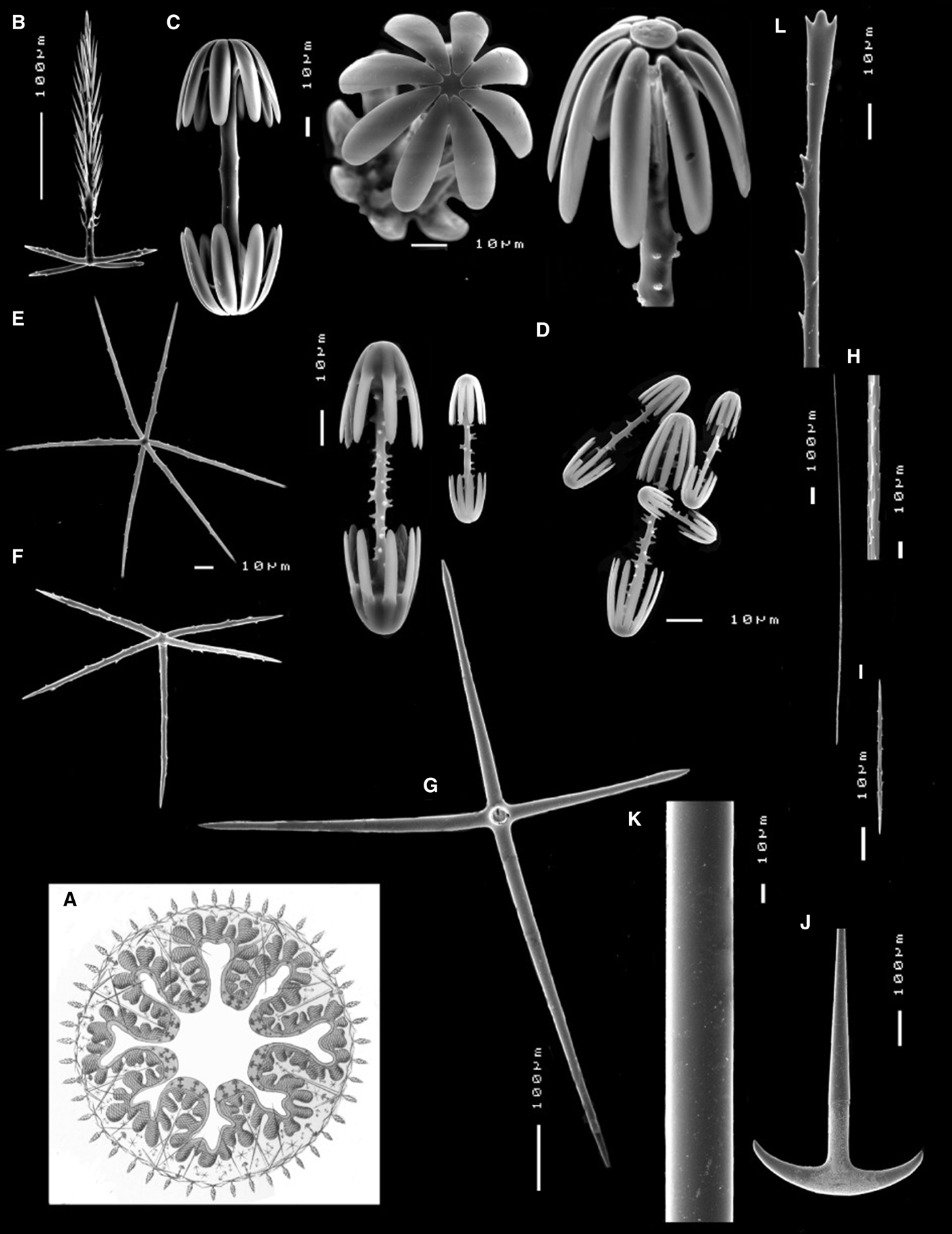
Dermal and atrial skeleton comprise pinular pentactines with tangential rays slightly curved and sometimes uneven in size, measuring 70-87-112 × 4-5-10 µm, and pinular rays measuring 172-265-448 × 7-9-10 µm (Figure 4B). Microscleres include macramphidiscs measuring 136-158-180 µm in length, with tuberculated shafts and different morphologies of the umbel (48-54-60 × 52-61-72 µm) (Figure 4C), and smaller mesa- and micramphidiscs with spined shafts and slightly overlapping size ranges (Figure 4D). Mesamphidiscs measure 68-102-140 µm in length with umbel measuring 23-37-52 µm (length) and 16-27-40 µm (width), whereas micramphidiscs measure 68-102-140 µm in length with umbel measuring 23-37-52 (length) and 16-27-40 µm (width). Microhexactines measure 68-88-112 × 3-4-5 µm (Figure 4E), whereas micropentactines (Figure 4F) and microstauractines measure 82-99-120 × 3-5-5 µm and 84-103-116 × 2-3-4 µm, respectively.

Fig. 4. Poliopogon amadou skeleton: (A) Illustration of a cross-section of P. amadou in Haeckel's Reference Haeckel1898 ‘Kunstformen der natur’; (B) pinular pentactine; (C) macramphidiscs; (D) mesa- and micramphidiscs; (E) microhexactine; (F) micropentactine; (G) choanosomal pentactine; (H) macrouncinate; (I) microuncinate; (J) anchor; (K) monaxone; (L) sceptre.
Choanosomal skeleton is composed of large smooth pentactines measuring 320-436-480 × 18-21-24 µm (Figure 4G), and three size-classes of uncinates. Most macrouncinates were broken but measure over 1.5 mm long (Figure 4H) whereas mesouncinates measure 460-776-980 × 2-3-4 µm. Some microuncinates were also found and these measure 50-118-216 × 1-1-2 µm (Figure 4I). Basalia is composed of two-toothed anchors (Figure 4J) and monaxones (Figure 4K) whereas the lateralia are sceptres (Figure 4L), all several mm long.
REMARKS
This species was originally illustrated and briefly described in Thomson (Reference Thomson1878) and further described in Schulze (Reference Schulze1887). Tabachnick & Menshenina (Reference Tabachnick, Menshenina, Hooper and Van Soest2002) examined the holotype and provided a detailed description and measurements of spicules. Our specimens perfectly match their description despite some variability in spicule dimensions. However, they report the presence of only two size-classes of uncinates – macro- and mesouncinates – whereas in our specimens we found also some, not very abundant, microuncinates. In our opinion these were either overlooked in the holotype or are simply a characteristic of this isolated population, thus bearing no specific taxonomic meaning.
DISTRIBUTION
The holotype of P. amadou (BMNH 1887.10.20.105) is a specimen collected from 2790 m depth southwest from the Canary Islands during the HMS Challenger expedition (station 3, 25°24′N 20°14′W). This species has also been reported from westernmost areas on the Mid-Atlantic Ridge between 2480–4022 m depth (Tabachnick & Menshenina, Reference Tabachnick, Menshenina, Hooper and Van Soest2002). In this study four specimens were collected from seamounts located south of the Azores archipelago: three specimens at the Great Meteor seamount between 2675–2765 m depth and one specimen at the Closs seamount at 2761 m depth.
DISCUSSION
Poliopogon amadou is the only species of this genus to occur in the Atlantic, all other occurring either in New Caledonia (P. claviculus, P. micropentactinus and P. zonecus) or in Central Polynesia (P. maitai) at depths between 697 and 4270 m (Tabachnick, Reference Tabachnick and Shirshov1988; Tabachnick & Lévi, Reference Tabachnick, Lévi and Crosnier2000).
During the campaign that led to the present study several isolated specimens were seen, but in one of the Great Meteor seamount stations (L09D12) a large number of specimens, of various sizes, was observed on a rocky outcrop (Figure 2A). Surprisingly, specimens were always found attached with their basal tuft to hard rocky substrate (Figure 2C) and never to the surrounding sandy substrate.
Due to data limitation (no videos available) it is beyond the scope of the present article to provide estimates of population size. However, from the pictures taken and observations made during the ROV dives it is possible to describe some of its characteristics. The population exhibited a very patchy distribution with local densities attaining up to 5 ind. m−2. The same pattern has been found in Pheronema carpenteri populations located on the Porcupine Seabight where densities varied from 0.8 to 5 ind. m−2 (Rice et al., Reference Rice, Thurston and New1990), and off the Moroccan slope where densities ranged from 0.17 ind. m−2 over 100 m transects to 6 ind. m−2 in single pictures covering 1.5 m2 (Barthel et al., Reference Barthel, Tendal and Thiel1996); and in Nodastrella asconemaoida in the Rockall Bank where densities varied from 0.66 to 6 ind. m−2 (Van Soest et al., Reference Van Soest, Van Duyl, Maier, Lavaleye, Beglinger, Tabachnick, Custódio, Lôbo-Hajdu, Hajdu and Muricy2007). These highly patchy distributions are likely a combination between suitable ecological conditions and local recruitment.
The fact that specimens here examined were collected at a depth very similar to that of the holotype and of the other available records may indicate that this species forms bathymetrically constrained band-shaped populations as found in other ground-forming hexactinellids such as Pheronema carpenteri (Barthel et al., Reference Barthel, Tendal and Thiel1996). Rice et al. (Reference Rice, Thurston and New1990) and White (Reference White2003) posited that the aggregation of Pheronema carpenteri in the Porcupine Seabight occurred close to, but not within, areas of enhanced bottom tidal currents, i.e. in areas with increased deposition of organic particulate load.
Many small individuals were observed recruiting at the base of larger individuals, which indicates a stable population. The same was observed in a large population of Vazella pourtalesii, known as the Russian hat on the Scotian shelf in the North-west Atlantic (Fuller, Reference Fuller2011). Whether these young specimens are the result of asexual propagation or of sexual reproduction remains unclear, as both have been reported in different species (Leys et al., Reference Leys, Mackie and Reiswig2007). Lastly, no dead individuals were observed which could indicate a relatively young population.
ACKNOWLEDGEMENTS
The authors warmly thank the Task Group for the Extension of the Continental Shelf (EMEPC, Portugal), in particular Professor Manuel Pinto de Abreu, for the opportunity to participate in their numerous sampling campaigns. We are also indebted to all the crew members of the NRP Almirante Gago Coutinho and colleague researchers onboard for their seamanship. Elly Beglinger (NCB Naturalis), Luisa Ribeiro (EMEPC) and Raquel Pereira are thanked for providing help with the SEM and figures, whereas Marte Torkildsen (University of Bergen) is acknowledged for helpful discussions regarding skeletal variability in hexactinellids.
FINANCIAL SUPPORT
This research was partly funded by a grant from the Global Census of the Marine Life on Seamounts (CenSeam) to J.R. Xavier and R.W.M. van Soest and by a postdoctoral grant (SFRH/BPD/62946/2009) from the Portuguese Science and Technology Foundation to J.R. Xavier.