Introduction
Grey seals (Halichoerus grypus) are a carnivorous pinniped that inhabit continental shelf regions of the North Atlantic Ocean. Their global population is estimated to be 632,000 animals of which half are mature individuals. There are two genetically distinct groups in the North-west Atlantic including 250,000 adults and another in the North-east Atlantic with 66,000 adults (Bowen, Reference Bowen2016).
Grey seals are protected by the Bern Convention 1979 as a vulnerable migratory species that constitutes a natural heritage asset to be preserved for future generations. Protection in European waters is delivered through the European Union Habitats Directive 1992 (Annex II and V), which requires European Marine Sites (EMS) such as Special Areas of Conservation (SAC) to be established typically to protect their breeding habitat (Davies, Reference Davies, Davies, Baxter, Bradley, Connor, Khan, Murray, Sanderson, Turnbull and Vincent2001). The UK hosts 34% of the world's grey seal population on the basis of pup production (SCOS, 2017) and as such has a key role to play in the conservation of this species. The UK has a special responsibility to protect this species (JNCC, 2016a, 2016b). SAC designation for grey seals in the UK includes the largest breeding colonies and coverage of the geographic range of the species (JNCC, 2014). In the UK area of the Celtic Sea there are five SACs with grey seals as a feature – Lundy and the Isles of Scilly in south-west England as well as Pembrokeshire Marine (Figure 1), Llyn Peninsula and Cardigan Bay in Wales (not shown). A further SAC offers protection to grey seals in north-west France (Parc Naturel Marin d'Iroise; Figure 1). There are additional SACs for grey seals in Ireland. In England, the Department for the Environment, Food and Rural Affairs (DEFRA) is establishing a network of designated Marine Protected Areas (MPAs) and has begun addressing the needs of mobile marine species within the process. Grey seals have yet to be considered under this framework in England (JNCC, 2016b; Edwards & Batey, Reference Edwards and Batey2017). In Cornwall, seals are offered protection under national legislation by the Conservation of Seals Act (1970) and Sites of Special Scientific Interest (SSSI) designation through the Wildlife and Countryside Act 1981 (as amended). SSSIs incorporate intertidal haul-out habitat above the mean low water mark. Where listed in the SSSI citation, it is an offence to damage, disturb or destroy seals (JNCC, 1989). In Cornwall, the Godrevy to St Agnes and Boscastle to Widemouth SSSIs have grey seals included within their citations.
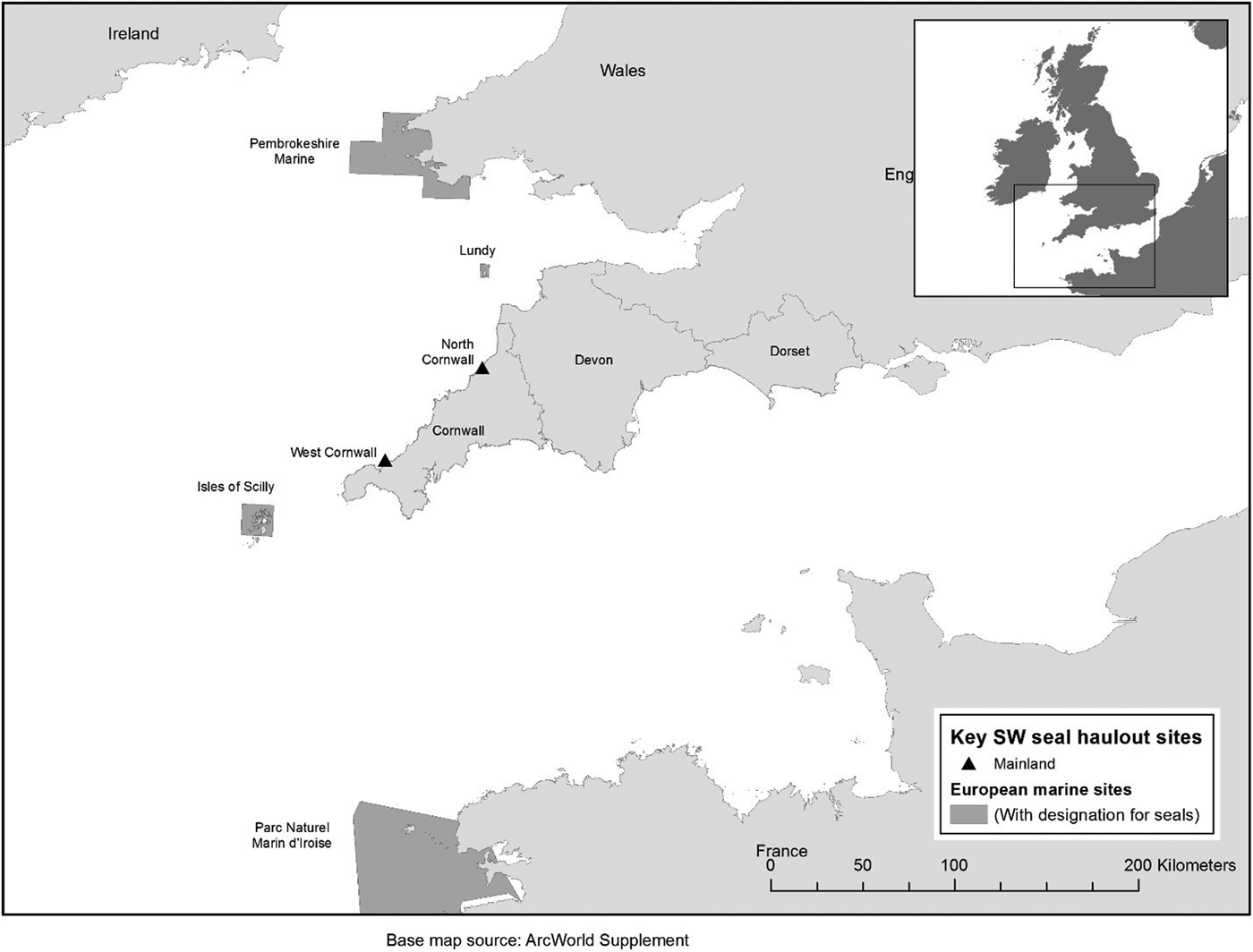
Fig. 1. European Marine Sites (Special Areas of Conservation) in south-west Wales, south-west England and north-west France with two SSSIs in Cornwall – all designated for seals.
As a mobile marine species, grey seals spend more than 80% of their time at sea and 90% of that below the surface (Harrison et al., Reference Harrison, Buckland, Thomas, Harris, Pomeroy and Harwood2006). Despite their mobility, they also demonstrate site fidelity, visiting the same predictable seasonal terrestrial sites to haul-out and reproduce (Gerondeau et al., Reference Gerondeau, Barbraud, Ridoux and Vincent2007).
In the UK, grey seals in Scotland may be geographically isolated from those in the Celtic Sea region (SCOS, 2013) despite long-range movements between these areas being reported (Vincent et al., Reference Vincent, Huon, Caurant, Dabin, Deniau, Dixneuf, Dupuis, Elder, Fremau, Hassani, Hemon, Karpouzopoulos, Lefeuvre, McConnell, Moss, Provost, Spitz, Turpin and Ridoux2017). In the Celtic Sea (which includes Cornwall, Devon, Wales, France and Ireland) short-term movements of grey seals have been reported between locations on the east and south-east coast of Ireland and south-west Wales (Kiely et al., Reference Kiely, Ligard, McKibben, Connolly and Baines2000), north and south-west Wales (SCOS, 2013) and between France and the Isles of Scilly, Cornwall, Wales and the Channel Isles (Vincent et al., Reference Vincent, Fedak, McConnell, Meynier, Saintjean and Ridoux2005; Huon et al., Reference Huon, Jones, Matthiopoulos, McConnell, Caurant and Vincent2015).
The distribution and movements of grey seals have been studied using a variety of techniques including tags, brands, paint dye, photogrammetry and satellite telemetry (Walker et al., Reference Walker, Trites, Haulena and Weary2011). Telemetry studies have revealed that adult grey seals can make repeated journeys of over 100 km between haul-out and foraging sites (McConnell et al., Reference McConnell, Chambers, Nicholas and Fedak1992, Reference Mcconnell, Fedak, Lovell and Hammond1999; Vincent et al., Reference Vincent, Fedak, McConnell, Meynier, Saintjean and Ridoux2005; SCOS, 2013) and foraging trips can last anywhere between 1 and 30 days (SCOS, 2013), covering as much as 75–100 km per day (McConnell et al., Reference Mcconnell, Fedak, Lovell and Hammond1999). While these studies provide detailed information on movements and foraging behaviour at the individual level, the large-scale application of this technology is limited by high costs (Karlsson et al., Reference Karlsson, Hiby, Lundberg, Jüssi, Jüssi and Helander2005). Satellite tag deployment durations typically last a few months and end during the grey seal annual moult when transmitters glued to their fur are shed (Sharples et al., Reference Sharples, Moss, Patterson and Hammond2012). In addition, capture and attachment procedures can be invasive, may cause disturbance at haul-out sites and can have hydrodynamic costs for tagged individuals (Hazekamp et al., Reference Hazekamp, Mayer and Osinga2009).
Photo identification (PID) is an effective (Wursig & Jefferson, Reference Wursig and Jefferson1990; Kaufman et al., Reference Kaufman, Coughran, Allen, Burns, Burton, Castro, Constantine, Franklin, Franklin, Forestell and Gales2011; Beck et al., Reference Beck, Foote, Kotter, Harries, Mandleberg, Stevick, Whooley and Durban2014) and minimally invasive research technique (Karlsson et al., Reference Karlsson, Hiby, Lundberg, Jüssi, Jüssi and Helander2005; Hiby et al., Reference Hiby, Lundberg, Karlsson, Watkins, Jüssi, Jüssi and Helander2007; Marshall & Bennett, Reference Marshall and Bennett2010) resulting in limited or no disturbance to the target species (Wursig & Jefferson, Reference Wursig and Jefferson1990; Thompson & Wheeler, Reference Thompson and Wheeler2008). PID can generate a range of information, including insights into distribution, abundance, habitat use, movements, life history and behaviour (Loughlin et al., Reference Loughlin, Cunningham, Gales, Wells, Boyd, Boyd, Bowen and Iverson2010; Macleod et al., Reference Macleod, Du Fresne, Mackey, Boyd and Mundie2010). Where the proportion of identifiable individuals is known, estimates of total abundance, survival and seasonal emigration/immigration can be studied by Capture–Mark–Recapture techniques (Loughlin et al., Reference Loughlin, Cunningham, Gales, Wells, Boyd, Boyd, Bowen and Iverson2010; Macleod et al., Reference Macleod, Du Fresne, Mackey, Boyd and Mundie2010).
If undertaken as part of a long-term structured research programme, PID can allow longitudinal studies of individuals over a lifetime of reproductive output, aid an assessment of cumulative threats and provide insights into individual behaviour (Loughlin et al., Reference Loughlin, Cunningham, Gales, Wells, Boyd, Boyd, Bowen and Iverson2010; Macleod et al., Reference Macleod, Du Fresne, Mackey, Boyd and Mundie2010). PID has been used on a range of marine species in the North-east Atlantic to track long-term movements and residency behaviour for bottlenose dolphins (Tursiops truncatus) (O’ Brien et al., Reference O'Brien, Berrow, Ryan, McGrath, O'Connor, Giovanna, Burrows, Massett, Klotzer and Whooley2009), killer whales (Orcinus orca) (Beck et al., Reference Beck, Foote, Kotter, Harries, Mandleberg, Stevick, Whooley and Durban2014) as well as grey seals in the Baltic Sea (Karlsson et al., Reference Karlsson, Hiby, Lundberg, Jüssi, Jüssi and Helander2005), Ireland (Kiely et al., Reference Kiely, Ligard, McKibben, Connolly and Baines2000), Wales (Kiely et al., Reference Kiely, Ligard, McKibben, Connolly and Baines2000; Beaumont & Goold, Reference Beaumont and Goold2007; Boyle, Reference Boyle2011; Langley et al., Reference Langley, Rosas, Hiby, Morris, Stringell and Pomeroy2018) and France (Gerondeau et al., Reference Gerondeau, Barbraud, Ridoux and Vincent2007).
Grey seals are ideal candidates for PID as they are long lived, relatively large, conspicuous and can be individually identified using natural fur patterns and scars (Wursig & Jefferson, Reference Wursig and Jefferson1990). Unique fur patterns remain legible and stable throughout their lives (Figure 1) particularly for adult and juvenile female grey seals (Paterson et al., Reference Paterson, Redman, Hiby, Moss, Hall and Pomeroy2013). The darkening of the adult male fur, combined with scarring from fighting with conspecifics, may however reduce the chances of re-identifying individuals (Paterson et al., Reference Paterson, Redman, Hiby, Moss, Hall and Pomeroy2013).
Public involvement in science programmes can yield considerable benefits relative to the resources committed (Prince, Reference Prince1993; Witt et al., Reference Witt, Penrose and Godley2007). The digital age has created revolutionary tools for PID from photography and image manipulation, email and social media to mobile recording applications and online data management platforms enabling organizations to engage and interact with large numbers of people (Davis et al., Reference Davis, Nibbelink and Howard2012; Zenetos et al., Reference Zenetos, Koutsogiannopoulos, Ovalis and Poursanidis2013; Gemmell et al., Reference Gemmell, McInnes, Heinrichs and Wijeyeratne2015).
Naturalists are keen to engage with the environment and wildlife (Silvertown, Reference Silvertown2009; Davies et al., Reference Davies, Bell, Bone, Head, Hill, Howard, Hobbs, Jones, Power, Rose, Ryder, Seed, Stevens, Toumi, Voulvoulis and White2011) and in south-west England they have provided an opportunity to improve knowledge on the distribution, movements and welfare status of grey seals. Increasing knowledge about grey seal movements in south-west England is particularly important given reported levels of fisheries impact on seals (Allen et al., Reference Allen, Jarvis, Sayer and Mills2012; Northridge et al., Reference Northridge, Kingston and Thomas2014, Reference Northridge, Kingston and Thomas2016) and increasing interests in marine spatial planning for UK and European waters. Furthermore, the wide-scale survey and PID efforts delivered by citizen scientists will likely increase opportunities to more coherently document links between coastal regions and marine/terrestrial protected areas. This is important as insights into connectivity could inform effective species management.
One of many challenges for the management of mobile species with predictable linkages (connectivity) between known areas is that conservation success can depend on the condition of sites outside of designated areas (Runge et al., Reference Runge, Martin, Possingham, Willis and Fuller2014). Given the geographic location of the UK's south-west peninsula (Figure 1), Cornwall may have an important role to play for seals moving between France, Wales and Ireland.
In this study, we use novel PID information from south-west England to demonstrate the interconnectivity of designated SACs, MPAs and SSSIs with multiple non-designated sites across the Celtic sea. This will provide a more coherent understanding of long-term grey seal movements across south-west England and beyond to inform ongoing marine spatial planning and management.
Materials and methods
In 2000, the Cornwall Seal Group Research Trust (CSGRT) began a long-term research programme to construct a comprehensive PID catalogue of grey seals at the West Cornwall site, one of two key mainland haul-out sites in south-west England – the other being in North Cornwall (Leeney et al., Reference Leeney, Broderick, Mills, Sayer, Witt and Godley2010) (Figure 2). Now, CSGRT's PID catalogue includes data from 54 haul-out sites (featuring grouped locations when in close proximity) in south-west UK (40 in Cornwall, one in the Isles of Scilly, nine in Devon, three in Wales and one in Dorset). PID catalogues used in this study were developed independently by the RSPB (Ramsey Island), The Wildlife Trust of South and West Wales (Skomer Island) in the Pembrokeshire Marine SAC, The Landmark Trust (Lundy Island) in the Lundy SAC and Dorset Wildlife Trust (Figure 1). Additional computer-aided EIRPHOT and SMRUPHOT catalogues have been developed in the region in conjunction with Natural Resources Wales and the Sea Mammal Research Unit using Extract Compare software but these were not used in this study (Hiby & Lovell, Reference Hiby and Lovell1990).

Fig. 2. Examples of pelage patterns from two long-term photo-identified seals. (A) Male grey seal ‘Hook’ identified 210 times at three sites observed from both left- and right-hand sides of the animal in 2000 and 2016 respectively. (B) Female grey seal ‘Carousel’ identified 130 times at four sites observed from both left- and right-hand sides of the animal in 2000 and 2016. Examples of key pelage patterns are highlighted.
Photographs of grey seals were taken during systematic, boat-based, coastal transect surveys (12 times a year) and from cliff top, land-based surveys by volunteers, non-governmental organizations (Coastwatch, Cornwall Wildlife Trust, National Trust and RSPB), local marine conservation groups and commercial marine ecotour operators across the region. PID events were variable through time and space, with some contributors from key haul-out sites providing frequent and repeated photographs (daily to once a month), while others were more sporadic. Context photos (zoomed out to show location) were taken of all seals to enable retrospective verification of data, then photographs were taken of individual seals from a variety of angles for PID, as permitted by safe coastal access points.
All photographs were subject to quality control by ascertaining presence of meta data, including location, date and photo quality. Every photo submitted and processed into a survey event album was screened by a single PID database coordinator. The lowest quality photos that were too distant or blurry were discarded. All remaining photos were manually compared visually to the PID catalogue from side by side photos in each album. PID survey albums and photographs were stored in digital archives as independent data collection events (by date, location and surveyor) to enable independent, retrospective verification. The PID catalogue includes representative photos of individual seals of known sex with clear re-identifiable markings alongside key word descriptions of dominant or visible fur patterns (on different regions of each animal, from both sides, from a variety of viewing angles and under a range of conditions, for example when wet and dry or moulting). Sex was determined based on fur pattern, head size/shape, behaviour and genitalia where visible.
Confirmed identifications of seals from the catalogue occurred when at least five different exact fur patterns on the same seal could be matched in the same relative positions (e.g. Figure 2) by the same PID coordinator and moderated by at least one other surveyor trained and experienced in PID. An identification was rejected if either the PID coordinator or moderator was in doubt (for example when five pattern matches were seen, but the rest of the seal's pattern appeared inconsistent or was masked by substrate). Where seals were not immediately recognized by eye, the catalogue was searched using key word descriptions of fur pattern shapes (for example letters or pictures visualized in the patterns) or scarring. Seals that remained unidentified were added to the PID catalogue as new seals where their sex was known and their pattern considered re-identifiable. This approach enabled their future identification or cross matching (when a single seal had been added to the catalogue more than once), although the possibility of one individual being represented by another remains (Hiby et al., Reference Hiby, Paterson, Redman, Watkins, Twiss and Pomeroy2013). Observation dates, locations, unique PID codes and supplementary survey data were entered into a custom built sightings database.
For the purposes of this paper, seals identified at multiple sites were termed multisite recaptures and were plotted in a matrix of sites against sites (Kiely et al., Reference Kiely, Ligard, McKibben, Connolly and Baines2000). Resulting site links were mapped in ArcMap (ESRI, Redmond, USA). Differences in the number of sites visited by males and females were tested (Mann–Whitney) within the statistical package R (R Core Team, 2014) and individual seal movement maps were created. Sites at which seals were identified were spatially cross-referenced to existing Marine Protected Areas (Figures 3–5).

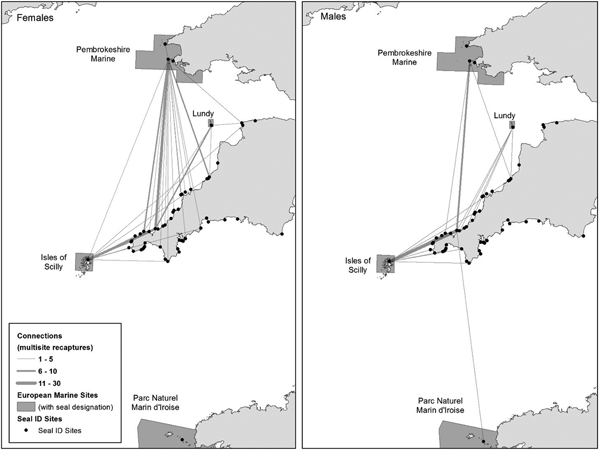
Fig. 3. All multisite recapture PID connections to/from the south-west UK. Circle size proportional to the number of PID connections per site. Line width represents the number of multisite recaptures between sites. Location of European Marine Sites (Special Areas of Conservation) with PID connections annotated.

Fig. 4. European Marine Sites (Special Areas of Conservation) multisite recapture connections with non-designated sites across south-west England. Female (N = 31) and male (N = 19) line width represents the number of multisite recapture connections between sites. Each link constitutes a single inferred movement for an individual seal resulting from PID.

Fig. 5. The UK Marine Protected Area Network (with or without designation for seals) in relation to the 54 haul-out sites where multisite recaptures were identified.
Results
Public engagement with CSGRT grew from 29,084 photo submissions (337 surveys) in 2008 to 78,527 photos (1537 surveys) in 2014. Photographs were submitted from 54 haul-out sites (between 2004 and 2014) ranging from south-west Wales (most northerly records) to northern Brittany, France (most southerly records). Reports originated from 154 members of the public, ranging from ad hoc single sightings to repeat surveyors following standardized protocols and from 18 public and voluntary organizations, 11 commercial marine ecotour companies, 28 contacts via social media websites and from seven systematic, boat-based seal PID projects.
The percentage of positively identified seals compared with all seals sighted varied from a minimum of 27% (N = 5332) at West Cornwall to a maximum of 82% (N = 773) at Lizard South. A total of 477 multisite recaptures were identified across multiple sites up to 230 km apart (Figure 3). The majority of these seals were identified at two sites (N = 346), whilst some were also seen at three sites (N = 87), four sites (N = 33), five sites (N = 8), six sites (N = 2) and seven sites (N = 1). Multisite recaptured seals created 1144 geographic linkages between the 54 haul-out sites at which seals were identified – of these, 16 sites were visited by 10 or more seals over the 16-year study period (Table 1). Each of these 16 sites was linked with between five and 41 other locations. All seals identified at more than one site were assigned sex (54% female, 46% male). In comparison, the sex class for all adult sightings recorded at all sites was 36% female and 64% male. Seven seals first identified in 2000 were resighted in 2015 of which four were males. Thirteen seals (10 males and three females) were identified as having died based on database matches made of dead seals photographed by Cornwall Wildlife Trust's Marine Strandings Network.
Table 1. Sites with 10 or more multisite captures (in rank order) 2000 to 2016

Four SACs in the region (Pembrokeshire, Lundy, Isles of Scilly and the Parc Naturel Marin d'Iroise) were visited by 104 identified seals from Cornwall (66 females and 38 males), linking these SACs to a total of 26 non-designated sites (Table 2). Two adult female seals were recaptured in two SACs – the Isles of Scilly and Pembrokeshire Marine. Seals were recaptured in 13 MPAs (Figure 5) and within two SSSIs where grey seals are protected – Godrevy to St Agnes SSSI (West Cornwall haul-out site) and Boscastle to Widemouth SSSI (North Cornwall haul-out site) (Figure 1).
Table 2. Links between European Marine Sites (Special Areas of Conservation) and other seal sites

The most connected West Cornwall site was linked to 41 other sites including four SACs by 379 seals and had been studied for the longest duration (16 years). The second most connected haul-out site in south-west England at North Cornwall (Leeney et al., Reference Leeney, Broderick, Mills, Sayer, Witt and Godley2010) was linked by 70 seals to 18 other sites including three SACs (Isles of Scilly, Lundy and Pembrokeshire Marine). Seals from the West Cornwall site were resighted up to 180 km north to Ramsey Island in Wales, 110 km north-east to Morte Point in north Devon, 190 km south-east to Start Point in south Devon and 230 km south to the Parc Naturel Marin d'Iroise in north-west France (Figure 3).
Both male and female seals from the PID catalogue moved between SACs and non-designated sites, but only male seals were resighted in all four SACs (Figure 4). The number of sites visited by female and male seals showed no statistically significant difference (Mann–Whitney, P = 0.05), with females visiting a similar number of sites (median 2, mean 2.4, SD 1 site) to males (median 2, mean 2.3, SD 1 site). Most (66%) of the 44 seals visiting four or more sites were female.
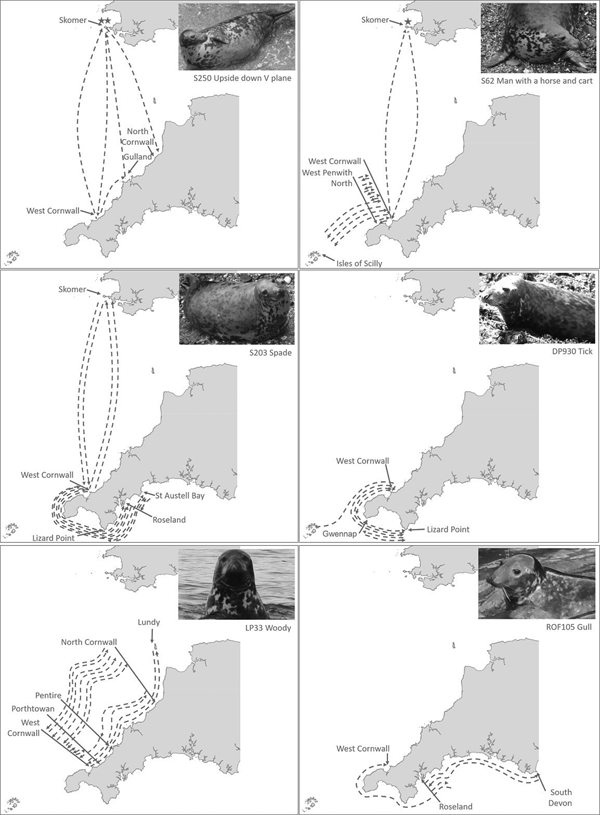
Ranging behaviour for individual seals differed, with some seals resighted between the same locations over time (Figure 6B–E) whilst others had more variable patterns of resightings and movement, visiting multiple locations (Figure 6A and F).

Fig. 6. Examples of individual seal movement maps as reconstructed by PID. Each arrow's start and end indicates at least one PID event. Arrows indicate inferred movements between sites. Stars indicate location of site where pupping was observed.
Discussion
Data contributed by citizen scientists have provided considerable insight into the movements of male and female grey seals reported here, over a regional scale for longer time periods than possible through satellite tagging (Walker et al., Reference Walker, Trites, Haulena and Weary2011; Jones et al., Reference Jones, McConnell, Sparling and Matthiopoilos2013) and at no financial cost. PID, utilizing manual matching of fur patterns, facilitated by key word searching, led to a knowledge base of individual seal movements from a previously unreported network of seal sites across Cornwall, the Isles of Scilly, Devon and beyond. Although PID effort at SACs was the most variable, sporadic and opportunistic, the results highlight the interconnectivity of SACs and multiple non-designated seal haul-out sites.
Most PID projects have focused on female grey seals (e.g. Karlsson et al., Reference Karlsson, Hiby, Lundberg, Jüssi, Jüssi and Helander2005; Hiby et al., Reference Hiby, Lundberg, Karlsson, Watkins, Jüssi, Jüssi and Helander2007; Paterson et al., Reference Paterson, Redman, Hiby, Moss, Hall and Pomeroy2013; Langley et al., Reference Langley, Rosas, Hiby, Morris, Stringell and Pomeroy2018) due to their distinctive patterns. Male grey seals also have fur patterns but with fewer, simpler markings (Vincent et al., Reference Vincent, Meynier and Ridoux2001; Paterson et al., Reference Paterson, Redman, Hiby, Moss, Hall and Pomeroy2013), providing contrasting challenges between the sexes for PID. Over two-thirds of grey seals in Cornwall are thought to be male (Leeney et al., Reference Leeney, Broderick, Mills, Sayer, Witt and Godley2010; Sayer, Reference Sayer2013, Reference Sayer2016). However, slightly more female seals were identified in our catalogue, suggesting it was likely that males were potentially under-identified.
Survey effort was considerable but variable, ranging from a minimum of once a month at some locations to daily throughout the year at one site enabling individual capture histories, site fidelity, productivity, behaviour and indicators of health (such as wound development) to be documented. The challenge of variable PID effort means opportunities to identify individuals between site surveys will inevitably have been missed, leading to false negatives (seals being present between surveys so not identified in addition to seals who were present but photographed from an unidentifiable angle or masked by substrate). Moderation of all seal identifications using the same rigorous PID coordinator alongside at least one other experienced PID researcher minimized errors in matching and means that the chance of false positives (two seals being incorrectly matched) is likely low and false negatives minimized. Although manual matching PID has been used successfully elsewhere with large data sets (Koivuniemi et al., Reference Koivuniemi, Auttila, Niemi, Levänen and Kunnasranta2016), results should always be interpreted with caution. As the chance of an identification and probability of a resighting were not equal at all sites, Capture–Mark–Recapture techniques could not be used robustly for estimating abundance from PID (Gerondeau et al., Reference Gerondeau, Barbraud, Ridoux and Vincent2007). Further, the technique does not allow complete reconstruction of movements made by seals as captures can only occur at surveyed sites. Future seal connectivity research would benefit from an integrated international approach through the sharing of PID catalogues and the amalgamation of different PID systems.
PID has contributed valuable insights into the interconnectivity of sites used by individual seals linked to south-west England. The maximum distance between identifications of a grey seal was up to 230 km coastal distance between sites where the seal was identified, which is within limits recorded in other parts of their range (125 to over 400 km in north-east Scotland) (Thompson et al., Reference Thompson, McConnell, Tollit, MacKay and Hunter1996; SCOS, 2012).
Knowledge about the variability of individual seal movements revealed by satellite telemetry (SCOS, 2012) has been reinforced by PID. Seal movements were complex between designated and non-designated sites, combining elements of both site fidelity (Kiely et al., Reference Kiely, Ligard, McKibben, Connolly and Baines2000) and migration (Jones et al., Reference Jones, McConnell, Sparling and Matthiopoilos2013). This highlights the challenge faced by policymakers and managers as reported seal identifications reveal links from four SACs to non-designated habitat where seals are not protected. Most SACs are used in multiple ways by people and managed with a narrow set of habitats or species-specific conservation objectives, but with no specific focus on the ecological function of the site (Rees et al., Reference Rees, Sheehan, Jackson, Gall, Cousens, Solandt, Boyer and Attrill2013). Wider application of the concepts of ‘functional linkage’ and ‘site integrity’ could provide an ecologically coherent network of protection for grey seals linked to SACs and SSSIs designated for seals whilst they visit non-designated habitat at this southern end of their range (Rees et al., Reference Rees, Sheehan, Jackson, Gall, Cousens, Solandt, Boyer and Attrill2013).
More recent marine designations exist in England and Wales in the form of Marine Protected Areas (MPAs). Seals were identified within 13 MPAs (Figure 5). Management regimes for these MPAs could benefit seals, for example by focusing on: the licensing of recreational activity; limits to fishing activity; and lost gear clean-up schemes within MPA boundaries to potentially reduce disturbance, by-catch and entanglement – three key impacts of human activity on seals (Wilson, Reference Wilson2005; Allen et al., Reference Allen, Jarvis, Sayer and Mills2012; Northridge et al., Reference Northridge, Kingston and Thomas2014; Sayer, Reference Sayer2015). Additional wider measures could include management limits on gill, tangle and trammel nets heavily implicated in seal by-catch in south-west England (Northridge et al., Reference Northridge, Kingston and Thomas2014, Reference Northridge, Kingston and Thomas2016) and tools to better assess cumulative impacts on grey seals. Such management measures would likely increase the survivorship of grey seals at sea where they spend 80% of their time, as well as at haul-out sites.
The key terrestrial moulting sites in south-west England had the highest number of multisite capture seals and the greatest number of linked sites (Table 1). Two of these sites at West Cornwall and North Cornwall fall within the Godrevy to St Agnes, and Boscastle to Widemouth SSSIs respectively. Increasing public awareness of SSSI legislation here could reduce disturbance impacts during the grey seal moulting season with associated benefits for all linked SACs.
Site connectivity revealed by this study suggest that parts of the grey seal annual life cycle are supported by different sites at different times of year. Seals at designated SAC pupping sites may need further protection at foraging and moulting sites (such as at West and North Cornwall) to form a network supporting all parts of their annual life cycle. In a complex adaptive ecosystem, impacts on seals at one undesignated site can lead to unpredictable effects across the network of sites connected by the impacted seals (Curtin & Prellezo, Reference Curtin and Prellezo2010) for example fisheries by-catch of pregnant females at one foraging site can reduce pup numbers at a distant SAC. The effect of eliminating one or more non-designated seal site (due to anthropogenic activities such as displacement by chronic disturbance) from the interconnected network of sites is unknown. What is more certain is that the conservation status of SACs in the Celtic and Irish Seas is at least partly dependent on what happens to its seals when they are outside the SAC boundaries in currently undesignated habitat.
Seals from the Isles of Scilly, Cornwall and Devon move across national and international borders and this behaviour has important implications for Marine Spatial Planning which aims to deliver ecosystem-based management. All important biological and ecological areas for grey seals will need to be considered, covering all stages of their life history for Marine Spatial Planning to benefit this species.
This study highlights that in the south-west UK there is a network of linked seal haul-out sites within a range of designated MPAs and beyond to non-designated locations which provide essential functional services for grey seals. The complex drivers and mechanisms involved in seal movements require considerably more study (Hays et al., Reference Hays, Ferreira, Sequeira, Meekan, Duarte, Bailey, Bailleul, Bowen, Caley, Costa, Eguíluz, Fossette, Friedlaender, Gales, Gleiss, Gunn, Harcourt, Hazen, Heithaus, Heupel, Holland, Horning, Jonsen, Kooyman, Lowe, Madsen, Marsh, Phillips, Righton, Ropert-Coudert, Sato, Shaffer, Simpfendorfer, Sims, Skomal, Takahashi, Trathan, Wikelski, Womble and Thums2016) but ensuring that protection follows grey seals as they move around the Celtic Sea and at the sites they inhabit is currently achievable through the application of functional linkage to SACs and SSSIs. This citizen science PID study provides evidence of site connectivity for the effective application of functional linkage, enabling interconnected designated and non-designated sites to be managed holistically.
Acknowledgements
CSGRT sincerely thank those who have shared photographs for seal identification and to those whose online photos have been used: T Adams, T Bain, S Beadle, A Bicknell, J Barry, C Bollen, S Bone, G Blundell, P Borecki, D Boyle, K Brown, B Buche, L Butters, C Caseldine, H Caseldine, R Castillo, D Chapman, R Chittenden, L Clarke, G Clegg, J Clegg, R Collins, C Cornishlight, C Curtis, R Daddow, M Dawton, D Drake, K Drake, S Duffield, R Durrant, J Dwyker, B Earll, D Faith, A Farr, E Farr, C Farran, M Fenwick, J Foster, K Francis, P Gillard, M Gregory, R Grey, D Groves, Sarah G, V Hall, D Haines, T Hardman, R Hartley, S Hassani, L Hawkes, G Hawkins, B Heaney, J Hirons, K Hiscock, T Hocking, K Hockley, C Hood, D Jarvis, D Jarvis, L Jarvis, D Jenkins, D Johnson, K Johnson, N Jones, C Jose, R Jutsum, C Keast, J Keast, C Keiser, J Kent, P King, C Lewis, I Lewis, Lizzie and John, K Lock, J Loveridge, J Loveridge, A Lowe, B Lowe, C Lowe, D McBride, B MacDonald, A Mills, C Mills, L Morgan, S Needham, F North, L Nutley, F Parrott, I Parrott, L Payne, B Phillips, N Ponting, S Pow, E Priestley, D Murphy, M Orman, T Pharic, J Rainbow, D Rail, L Rogers, J Ross, T Russell, L Sargeant, W Sargeant, G Savage, H Selley, P Semmens, C Spooner, D Spooner, E Stubbings, M Stephens, A Steele, S Stewart, M Taylor, S Taylor, T Thirlaway, M Thorne, N Tomalin, L Tozer, S Van Trijfel, C Waddell, P Walker, D Warmsley, G Waters, R Wells, P Welsh, K Wheston, P White, C Wilding, B Williams and J Williams.
CSGRT are grateful to collaborate with the following organizations: British Divers Marine Life Rescue; Cardigan Bay Marine Wildlife Centre; the Cornish Seal Sanctuary; Cornwall Wildlife Trust's Living Seas Team, Seaquest SW and Marine Strandings Network; the Landmark Trust and Lundy wardens; Morte Wildlife Group; National Coastwatch Institute; the National Trust; Natural England; Natural Resources Wales; Oceanopolis; Parc Natural Marin Iroise; Royal Society for the Protection of Birds; Royal Society for the Prevention of Cruelty to Animals Wildlife Hospitals; Wildlife Trusts from Devon and South and West Wales with Skomer wardens.
Thank you to the following ecotourism operators who contributed to this work: Atlantic Diving, AK Wildlife Cruises, Charles Hood, Coastline Ecocoasteers, Cornish Sea Tours, Dive Scilly, Elemental Tours, Koru Kayaking, Marine Discovery, Orca Sea Safaris and Scilly Seal Snorkelling.
Seal PID projects (PIPs – CASPIP, INTPIP, IOSPIP, LISPIP, MERIFIC, POLPIP, STAPIP) involving hundreds of volunteer surveyors have contributed hugely to our PID effort and have been organized by the following groups: CSGRT, Cornwall Wildlife Trust, Cornwall College, Looe Marine Conservation Group, National Trust, Polzeath Marine Conservation Group and the University of Exeter.
This work has relied on the inspiration, energy and enthusiasm of the CSGRT steering group, trustees, supporters, families (particularly Chris Howell) and friends underpinned by scientific capacity building provided by Cornwall College and the University of Exeter.
Financial support
This research received no specific grant and was undertaken by volunteers at no direct cost to CSGRT. This paper refers to PID using photos taken in part during projects funded by the Isles of Scilly Area of Outstanding Natural Beauty; University of Exeter; Natural England; Marine Energy in Far Peripheral and Island Communities (MERiFIC); Sea-Changers; Wave Hub; World Animal Protection and Patagonia.