INTRODUCTION
Sponges of the family Polymastiidae Gray, Reference Gray1867 have a simple spicule assortment which is usually limited to several size categories of smooth monactines (Boury-Esnault, Reference Boury-Esnault, Hooper and van Soest2002). However, in addition to these common spicules, some species also possess distally ornamented monactines. This additional category of spicules was first recorded in polymastiids by Sollas (Reference Sollas1882) who noticed the rounded swellings on the distal tips of projecting monactines in his new species Radiella schoenus from the Norwegian coast. Three years later Vosmaer (Reference Vosmaer1885) recorded similar spicules in his new species Polymastia capitata from the Arctic. Dendy & Ridley (Reference Dendy and Ridley1886) noted the similarity between R. schoenus and P. capitata relegating the latter to synonymy with the former. They also established a new genus, Proteleia, for their new species, P. sollasi from South Africa, which was distinguished by the grapnel-like distal ornamentations of its protruding spicules.
In 1898 Topsent erected two more polymastiid genera displaying ornamented monactines, Tylexocladus for his new species, T. joubini from Azores, which was notable for the denticulate distal ornamentations on its cortical spicules, and Sphaerotylus for Vosmaer's P. capitata, which was characterized by the spherical swellings on its projecting spicules. To identify these spicules with usual tyles on the proximal extremities and ornaments on the distal extremetities protruding above the sponge surface Topsent used the term exotyle introduced by him 2 years earlier (Topsent, Reference Topsent1896) for the similar spicules in Gomphostegia loricata (now Mycale (Rhaphidotheca) loricata, see Van Soest et al., Reference van Soest, Boury-Esnault, Hooper, Rützler, de Voogd, Alvarez de Glasby, Hajdu, Pisera, Manconi, Schoenberg, Janussen, Tabachnick, Klautau, Picton, Kelly, Vacelet, Dohrmann, Díaz and Cárdenas2015) from the family Mycalidae.
For the time being nine species of Sphaerotylus from various locations in polar and temperate waters of both hemispheres, two species of Proteleia from the southern hemisphere and two species of Tylexocladus, one from the North Atlantic and the other from the South Pacific are recognized as valid (Van Soest et al., Reference van Soest, Boury-Esnault, Hooper, Rützler, de Voogd, Alvarez de Glasby, Hajdu, Pisera, Manconi, Schoenberg, Janussen, Tabachnick, Klautau, Picton, Kelly, Vacelet, Dohrmann, Díaz and Cárdenas2015). Exotyles have also been recorded in Trachyteleia stephensi Topsent, Reference Topsent1928 and in two New Zealand species of Polymastia Bowerbank, Reference Bowerbank1864, P. tapetum Kelly-Borges & Bergquist, Reference Kelly-Borges and Bergquist1997 and P. umbraculum Kelly-Borges & Bergquist, Reference Kelly-Borges and Bergquist1997. Affinities between all these taxa have been discussed (Kelly-Borges & Bergquist, Reference Kelly-Borges and Bergquist1997; Boury-Esnault, Reference Boury-Esnault, Hooper and van Soest2002), but they have never been properly revised, and there is still no agreement on the differences at the generic level.
In this paper we review all known species and varieties of Proteleia, Sphaerotylus, Trachyteleia and Tylexocladus along with those species of Polymastia which display ornamented exotyles. We establish a new genus, Koltunia gen. nov. for the Antarctic species Proteleia burtoni Koltun, Reference Koltun, Pavlovskii, Andriyashev and Ushakov1964, describe three new species of Sphaerotylus – from South Africa, Ireland and West Greenland and propose the transfer of two South Pacific species of Polymastia, one to Sphaerotylus, the other to Proteleia. Finally, we reconsider the affinities of the species studied based on multiple morphological characters.
MATERIALS AND METHODS
This study was based on the type specimens and other material stored in Ulster Museum, Belfast (BELUM), Natural History Museum, London (BMNH), Göteborg Natural History Museum (GNM), Muséum National d'Histoire Naturelle, Paris (MNHN), Musée Océanographique de Monaco (MOM), Museum of New Zealand, Te Papa Tongarewa, Wellington (NZNM), National Museum of Natural History, Leiden (RMNH), Smithsonian National Museum of Natural History, Washington (USNM), Zoological Institute of Russian Academy of Sciences, Saint-Petersburg (ZIN RAS), Museum für Naturkunde, Berlin (ZMB), University Museum of Bergen (ZMBN) and Natural History Museum of Denmark, University of Copenhagen (ZMUC). Additional fresh material was collected from the Norwegian coast during cruises by the University of Bergen. The architecture of the sponge skeletons was examined under light microscope on histological sections prepared on a precise saw with a diamond wafering blade after embedding sponge fragments in epoxy resin as described by Boury-Esnault et al. (Reference Boury-Esnault, Marschal, Kornprobst and Barnathan2002), Vacelet (Reference Vacelet2006) and Boury-Esnault & Bézac (Reference Boury-Esnault, Bézac, Custódio, Lôbo-Hajdu, Hajdu and Muricy2007). Spicules were examined under light microscope and SEM after their isolation from organic matter in nitric acid following standard procedures. The number of specimens used for spicule measurements is given in the corresponding section of the description of each species. The number of spicules of each category measured in one specimen is indicated as N. Measurements are presented as minimum–mean–maximum, unless otherwise indicated.
SYSTEMATICS
Systematic index
Class demospongiae Sollas, Reference Sollas1885
Suborder heteroscleromorpha Cárdenas, Perez & Boury-Esnault, Reference Cárdenas, Perez and Boury-Esnault2012
Order polymastiida Morrow & Cárdenas, Reference Morrow and Cárdenas2015
Family polymastiidae Gray, Reference Gray1867
Genus Koltunia gen. nov.
K. burtoni (Koltun, Reference Koltun, Pavlovskii, Andriyashev and Ushakov1964) comb. nov.
Genus Proteleia Dendy & Ridley, Reference Dendy and Ridley1886
P. sollasi Dendy & Ridley, Reference Dendy and Ridley1886
P. tapetum (Kelly-Borges & Bergquist, Reference Kelly-Borges and Bergquist1997) comb. nov.
Genus Sphaerotylus Topsent, Reference Topsent1898
S. antarcticus Kirkpatrick, Reference Kirkpatrick1907
S. antarcticus drygalskii Hentschel, Reference Hentschel and von Drygalski1914
S. borealis (Swarczewsky, Reference Swarczewsky1906)
S. capitatus (Vosmaer, Reference Vosmaer1885)
S. exospinosus Lévi, Reference Lévi and Crosnier1993
S. exotylotus Koltun, Reference Koltun and Bogorov1970
S. isidis (Thiele, Reference Thiele1905) comb. nov.
S. raphidophora Austin, Ott, Reiswig, Romagosa & McDaniel, Reference Austin, Ott, Reiswig, Romagosa and McDaniel2014
S. renoufi sp. nov.
S. sceptrum Koltun, Reference Koltun and Bogorov1970
S. strobilis sp. nov.
S. tjalfei sp. nov.
S. vanhoeffeni Hentschel, Reference Hentschel and von Drygalski1914
S. verenae Austin, Ott, Reiswig, Romagosa & McDaniel, Reference Austin, Ott, Reiswig, Romagosa and McDaniel2014
Genus Trachyteleia Topsent, Reference Topsent1928
T. stephensi Topsent, Reference Topsent1928
Genus Tylexocladus Topsent, Reference Topsent1898
T. hispidus Lévi, Reference Lévi and Crosnier1993
T. joubini Topsent, Reference Topsent1898
Incertae sedis
Polymastia umbraculum Kelly-Borges & Bergquist, Reference Kelly-Borges and Bergquist1997
Description of taxa
Family polymastiidae Gray, Reference Gray1867
DIAGNOSIS
Sponges of massive, encrusting, globular, discoid or pedunculate growth form. Surface slightly velvety to very hispid. Choanosomal skeleton composed of radial megasclere tracts. A complex specialized cortical skeleton is developed to a greater or lesser degree, composed of at least a palisade of tylostyles, or oxeas and/or exotyles. Spicules comprise two or more size categories and include tylostyles, subtylostyles, strongyloxeas, styles or oxeas. Free spicules are always present in the choanosome; they may be intermediary or small tylostyles as well as various microscleres including smooth centrotylote microxeas, acanthose microxeas, raphides in trichodragmata and astrotylostyles. A fringe of long spicules is often present bordering the edge of the body where it is in contact with the substratum (from Plotkin & Janussen, Reference Plotkin, Janussen, Martínez Arbizu and Brix2008).
Genus Koltunia gen. nov.
TYPE SPECIES
Proteleia burtoni Koltun, Reference Koltun, Pavlovskii, Andriyashev and Ushakov1964 (designation herein).
DIAGNOSIS
Thickly encrusting sponges with shaggy surface. Main choanosomal skeleton composed of longitudinal tracts of large styles and subtylostyles. These tracts ascend forming cortical bouquets and a thick surface hispidation. Auxiliary choanosomal skeleton comprises free-scattered small tylostyles. Cortex and surface hispidation reinforced by small tylostyles and giant exotyles (several mm in length). Distal extremities of the exotyles with several long claws resembling grapnels.
ETYMOLOGY
Named after the late Dr Vladimir M. Koltun, the greatest Russian sponge expert of the 20th century who described the type species of this genus.
REMARKS
This new genus is established due to the unique ornamentations of its exotyles in combination with a single-layered cortex and two size categories of monactines. The single layered-cortex is recorded in some species of several polymastiid genera, but usually it is composed of a palisade of either small tylostyles (e.g. in Polymastia invaginata Kirkpatrick, Reference Kirkpatrick1907, Sphaerotylus raphidophora Austin, Ott, Reiswig, Romagosa & McDaniel, Reference Austin, Ott, Reiswig, Romagosa and McDaniel2014, Spinularia spinularia (Bowerbank, Reference Bowerbank1866) and Tentorium semisuberites (Schmidt, Reference Schmidt1870)) or exotyles (e.g. in Sphaerotylus exotylotus Koltun, Reference Koltun and Bogorov1970 and S. vanhoeffeni Hentschel, Reference Hentschel and von Drygalski1914) while in Koltunia the cortex is made of the bouquets of principal spicules with small tylostyles and exotyles embedded in between. The absence of intermediary size monactine category is typical of Weberella Vosmaer, Reference Vosmaer1885. Apart from this feature, there are no other similarities between Weberella and Koltunia.
Koltunia burtoni (Koltun, Reference Koltun, Pavlovskii, Andriyashev and Ushakov1964) comb. nov.
(Figures 1 & 2)

Fig. 1. Koltunia burtoni: (A) holotype ZIN RAS 10605, habitus; (B) fragment of the holotype BMNH 1986.7.9.6, habitus; (C) longitudinal section through the body of the holotype, general view; (D) the same section, detail of cortex. Scale bars: A–C, 5 mm; D, 0.5 mm.

Fig. 2. Koltunia burtoni, spicules: (A) principal subtylostyle, general view; (B) proximal tip of the subtylostyle depicted in A, detailed view; (C) distal tip of the subtylostyle depicted in A, detailed view; (D) small tylostyles; (E) proximal tip of an exotyle, detailed view; (F) the same exotyle, distal ornamentation, detailed view; (G) and (H) distal ornamentations of other exotyles, detailed view. Scale bars: A, 0.5 mm; B and C, 0.01 mm; D–H, 0.05 mm.
Original description: Proteleia burtoni Koltun, Reference Koltun, Pavlovskii, Andriyashev and Ushakov1964, p. 28, text- figure 4.
SYNONYMS AND CITATIONS
Proteleia burtoni (Koltun, Reference Koltun1976, p. 168; Kelly-Borges & Bergquist, Reference Kelly-Borges and Bergquist1997, p. 374; Boury-Esnault, Reference Boury-Esnault, Hooper and van Soest2002, p. 204).
TYPE MATERIAL
Holotype: ZIN RAS 10605 (specimen in alcohol and slides 6299, 11864, Figure 1A), BMNH 1986.7.9.6 (fragment of holotype in alcohol, Figure 1B), North of Balleny Islands, Southern Ocean, 64°03′S 161°59.2′E, 3000 m, RV ‘Ob’, station 57, 29.03.1956, coll. Ushakov and Belyaev.
DESCRIPTION
External morphology
Holotype – considerably damaged, ~ 1.9 × 1.3 × 0.5 cm in size, with shaggy dark-grey surface, without visible papillae (Figure 1A).
Skeleton
Main choanosomal skeleton composed of longitudinal tracts of principal spicules (Figure 1C). These tracts cross the cortex, where they expand into bouquets forming a 380–790 µm thick layer, and penetrate the surface, giving it a hirsute appearance (Figure 1D). Cortical bouquets reinforced by small spicules and giant exotyles. Auxiliary choanosomal skeleton comprises free-scattered small spicules.
Spicules
(N = 7 for exotyles, N = 10 for other categories)
-
• Principal spicules – straight or gently curved, slender or slightly fusiform styles to subtylostyles (Figure 2A–C). Length 1700–2488–3201 µm, diameter of tyle 14.2–16.6–18.5 µm, proximal diameter of shaft 13.5–14.9–17.9 µm, maximum diameter of shaft 23.8–26.5–29.3 µm. Koltun (Reference Koltun, Pavlovskii, Andriyashev and Ushakov1964) also recorded much longer principal spicules, up to 6000 µm. However, on the slides examined the spicules longer than 3200 µm were broken and therefore their length could not be estimated.
-
• Small spicules – straight, slender or slightly fusiform tylostyles (Figure 2D). Length 165–310–418 µm, diameter of tyle 5.9–6.5–7.1 µm, proximal diameter of shaft 3.3–4.0–5.0 µm, maximum diameter of shaft 6.0–8.0–10.0 µm. Koltun (Reference Koltun, Pavlovskii, Andriyashev and Ushakov1964) recorded small tylostyles from 150 to 550 µm in length.
-
• Exotyles flexuous and slender. Length 1900–3005–4300 µm, maximum diameter of shaft 24.0–33.2–40.0 µm. Exotyles may reach greater size, but the longest spicules were broken. Proximal extremities of the exotyles rounded, occasionally with weakly developed tyles (Figure 2E). Distal extremities ornamented with two to five curved or bent claws directed towards the proximal ends resembling the clads of anatriaenes in spirophorid and astrophorid sponges (grapnel-shaped). Each claw 37.9–59.2–80.0 µm long, divided into three to six processes at the tip. The claws may be symmetrically arranged (Figure 2F) or concentrated on one side of the shaft (Figure 2G, H).
OCCURRENCE
(Figure 3)

Fig. 3. Distribution of Polymastiidae with ornamented exotyles in the southern hemisphere: white crosses, Koltunia burtoni; white heart, Proteleia sollasi; white triangle, Proteleia tapetum; white stars, Sphaerotylus antarcticus; black star, Sphaerotylus antarcticus drygalskii; white square, Sphaerotylus isidis; white circles, Sphaerotylus vanhoeffeni, identification approved; black circles, Sphaerotylus vanhoeffeni, identification dubious; black trefoil, Polymastia umbraculum.
Southern Ocean: continental sectors 4 (off Sabrina Coast – Koltun, Reference Koltun1976) and 5 (off Balleny Islands – Koltun, Reference Koltun, Pavlovskii, Andriyashev and Ushakov1964) (sectors numbered according to Sarà et al., Reference Sarà, Balduzzi, Barbieri, Bavestrello and Burlando1992), 2267–3000 m.
REMARKS
Koltun (Reference Koltun, Pavlovskii, Andriyashev and Ushakov1964) placed his new species in Proteleia based on the grapnel-like distal ornamentations on the exotyles that were considered to be the main distinguishing feature of this genus (Dendy & Ridley, Reference Dendy and Ridley1886). Subsequent authors followed Koltun (Kelly-Borges & Bergquist, Reference Kelly-Borges and Bergquist1997; Boury-Esnault, Reference Boury-Esnault, Hooper and van Soest2002). However, the exotyles of the type species of Proteleia, P. sollasi, are in fact filiform spicules less than 600 µm long, with small distal ornamentations varying from irregularly grapnel-shaped to umbrelliform. These exotyles are sparsely scattered over the surface. Conversely, in K. burtoni the exotyles are thick and reach several millimetres in length. They are densely scattered over the sponge surface. Their distal ornamentations are large claws resembling the clads of anatriaenes, which is a unique feature among the polymastiids. Moreover, neither the external morphology, nor the cortical architecture, or the spicule assortment of K. burtoni bears any similarities with P. sollasi. The shaggy surface and large principal spicules of K. burtoni rather resemble those of Sphaerotylus borealis (Swarczewsky, Reference Swarczewsky1906), S. antarcticus Kirkpatrick, Reference Kirkpatrick1907 and Polymastia invaginata than the velvety surface and smaller spicules of Proteleia sollasi. A single-layered cortex of K. burtoni is similar to that of P. invaginata, although the cortex of the latter species comprises an ordinary palisade of small tylostyles overlapped by bouquets of principal spicules (Plotkin & Janussen, Reference Plotkin, Janussen, Martínez Arbizu and Brix2008), whereas in K. burtoni there is no palisade and single small tylostyles are embedded between the bouquets of large spicules. Conversely, the cortex of Proteleia sollasi comprises three layers, a superficial palisade of small tylostyles, an inner tangential layer of intermediary spicules and a palisade of intermediary spicules in between.
Genus Proteleia Dendy & Ridley, Reference Dendy and Ridley1886
TYPE SPECIES
Proteleia sollasi Dendy & Ridley, Reference Dendy and Ridley1886 (by monotypy).
DIAGNOSIS
Thickly encrusting sponges with velvety surface and papillae. Main choanosomal skeleton made of longitudinal tracts of principal spicules. Auxiliary choanosomal skeleton comprises free-scattered small and intermediary spicules. Cortex constituted by a superficial palisade of small spicules and an inner layer of tangentially arranged intermediary spicules, and reinforced by exotyles. In some species an additional palisade of intermediary spicules may be present between the superficial palisade and the inner tangential layer. Principal spicules are usually fusiform styles. Small and intermediary spicules are mainly tylostyles. Exotyles thin, shorter than 1 mm, with prominent distal ornamentations which may be umbrelliform, fungiform or grapnel-shaped with short protuberances on the edges.
Proteleia sollasi Dendy & Ridley, Reference Dendy and Ridley1886
(Figures 4 & 5)

Fig. 4. Proteleia sollasi, holotype BMNH 1887.5.2.62: (A) habitus; (B) unstained longitudinal section through the body, general view; (C) longitudinal section through the body stained with carmine, detail of cortical palisade; (D) longitudinal section through a papilla stained with carmine, general view; (E) the same section, detail of the papilla wall; (F) unstained transversal section through a papilla. Scale bars: A, 10 mm; B, 0.5 mm; C, 0.2 mm; D, 1 mm; E, 0.3 mm; F, 1 mm.

Fig. 5. Proteleia sollasi, spicules: (A) larger principal strongyloxea; (B) smaller principal strongyloxea; (C) intermediary subtylostyles; (D) small tylostyles; (E) exotyle with a prominent grapnel-like distal ornamentation, general view; (F) exotyle with a reduced distal ornamentation, general view; (G) proximal tip of the exotyle depicted in E, detailed view; (H) grapnel-like distal ornamentation of the exotyle depicted in E, detailed view; (I) proximal tip of the exotyle depicted in F, detailed view; (J) distal ornamentation of the exotyle depicted in F, detailed view. Scale bars: A, 0.1 mm; B, 0.04 mm; C and D, 0.02 mm; E and F, 0.1 mm; G–J, 0.004 mm.
Original description: Proteleia sollasi Dendy & Ridley, Reference Dendy and Ridley1886, p. 152, pl. 5.
SYNONYMS AND CITATIONS
Proteleia sollasi (Ridley & Dendy, Reference Ridley and Dendy1886, p. 488; Reference Ridley and Dendy1887 p. 214, pl. XLII figures 6–8, pl. XLIV figure 2; Von Lendenfeld, Reference von Lendenfeld and Schulze1903, p. 29; Kelly-Borges & Bergquist, Reference Kelly-Borges and Bergquist1997, p. 374, figure 5D–E; Boury-Esnault, Reference Boury-Esnault, Hooper and van Soest2002, p. 204, figure 3).
TYPE MATERIAL
Holotype: BMNH 1887.5.2.62 (specimen in alcohol and eight slides), BMNH 1891.10.3.95 (one slide prepared from holotype), BMNH 1891.10.3.96 (one slide prepared from holotype), Simon's Bay near the Cape of Good Hope, South Africa, SE Atlantic, 18–36 m (10–20 fathoms), expedition on RV ‘Challenger’ in 1873–1876.
DESCRIPTION
External morphology
Holotype cushion-shaped, detached from substratum, ~ 5 × 3 × 0.3 cm in size (Figure 4A). Surface velvety, covered by small amounts of debris and shell pieces, with 27 cylindrical or conical papillae up to 0.8 cm long and 0.4 cm in diameter at base. Both surface and papillae pale yellow in colour. Oscula not visible. Some papillae sectioned transversally demonstrating a central canal surrounded by numerous peripheral canals.
Skeleton
Main choanosomal skeleton composed of longitudinal tracts (~ 250 µm thick) of principal spicules which enter the cortex (Figure 4B). Auxiliary choanosomal skeleton comprises singly scattered intermediary and small spicules. Cortex consists of a superifical palisade (~ 150 µm thick) of small spicules, an inner tangential layer (300–500 µm thick) of intermediary spicules and a palisade (~ 350 µm thick) of intermediary spicules in between, the two palisades intermingling (Figure 4C). The superficial palisade reinforced by sparse exotyles. All three cortical layers stretch along the walls of papillae, but the boundary between the inner palisade and the tangential layer is not well defined (Figure 4D–F). Central exhalant canal surrounded by ascending choanosomal tracts (Figure 4F). Bulkheads between peripheral canals reinforced by intermediary spicules.
Spicules
(N = 8 for exotyles, N = 10 for other categories)
-
• Principal spicules – straight strongyloxeas or fusiform subtylostyles with weakly developed tyles (Figure 5A, B). Length 473–974–1200 µm, proximal diameter of shaft 6.7–8.0–9.2 µm, maximum diameter of shaft 15.0–28.0–37.6 µm.
-
• Intermediary spicules – gently curved, fusiform subtylostyles (Figure 5C). Length 191–206–240 µm, diameter of tyle 6.5–7.3–8.1 µm, proximal diameter of shaft 5.6–6.2–7.0 µm, maximum diameter of shaft 11.5–14.8–19.0 µm.
-
• Small spicules – straight or gently curved, slender tylostyles (Figure 5D). Length 125–152–180 µm, diameter of tyle 2.5–4.0–5.0 µm, proximal diameter of shaft 2.3–2.7–3.1 µm, maximum diameter of shaft 3.1–4.0–5.0 µm.
-
• Exotyles gently curved, slender, 350–463–555 µm long and 5.0–5.5–6.0 µm in diameter (Figure 5E, F). Their proximal extremities rounded, usually without tyles or more rarely with weakly developed tyles (Figure 5G, I). Distal ornamentations irregular, usually with four to eight more or less prominent short protuberances or claws directed towards the proximal tips, umbrelliform or occasionally grapnel-shaped (Figure 5H). Width of ornamentation with protuberances 4.0–4.9–6.3 µm. Some ornamentations with reduced protuberances and slightly displaced along the shafts (Figure 5J). Surface of ornamentations tuberculated or granulated.
REMARKS
Proteleia sollasi is known only from the holotype. The presence of an extra palisade of intermediary spicules in the cortex and grapnel-like ornamentations on the exotyles were considered as the main distinctive features of this species (Dendy & Ridley, Reference Dendy and Ridley1886; Boury-Esnault, Reference Boury-Esnault, Hooper and van Soest2002). Meanwhile, we have revealed that the shape of the exotyle ornamentations in P. sollasi is irregular and varies from grapnel-like to umbrelliform. Very similar exotyles are recorded in Proteleia tapetum (Kelly-Borges & Bergquist, Reference Kelly-Borges and Bergquist1997) and Polymastia umbraculum Kelly-Borges & Bergquist, Reference Kelly-Borges and Bergquist1997. Furthermore, irregular ornamentations with short protuberances are present on some exotyles of Sphaerotylus antarcticus and S. borealis, although their exotyles are much longer than those in Proteleia spp. Grapnel-like exotyle ornamentations with very long claws are typical of Koltunia burtoni, a species previously placed into Proteleia. However, its giant exotyles are several times larger than those of of P. sollasi. Moreover, K. burtoni is distinguished from Proteleia spp. by a single-layered cortex and a thick surface hispidation. The extra palisade layer in cortex has not been recorded in any other polymastiid with exotyles other than P. sollasi. But among other polymastiids Polymastia corticata Ridley & Dendy, Reference Ridley and Dendy1886 and P. littoralis Stephens, Reference Stephens1915 do have such an extra palisade of intermediary spicules lying under the superficial palisade of small spicules.
Proteleia tapetum (Kelly-Borges & Bergquist, Reference Kelly-Borges and Bergquist1997) comb. nov.
(Figures 35 & 36)
Original description: Polymastia tapetum Kelly-Borges & Bergquist, Reference Kelly-Borges and Bergquist1997, p. 372, figures 4 & 5A–C.
TYPE MATERIAL
Holotype: NZNM Por 65 (specimen in alcohol, a fragment studied), BMNH 1996.2.22.10 (fragment of holotype in alcohol, studied), Castor Bay, east Coast of North Island, New Zealand, 36°45′S 174°46′E, mid low-tide, 12.09.1988.
Paratype: NZNM Por 557 (one specimen, not studied), from the same sample as the holotype.
Paratype: NZNM Por 558 (one specimen, not studied), Goat Island, Leigh, New Zealand, 36°16′S 174°48′E, shallow subtidal, 08.03.1991.
DESCRIPTION
External morphology
(According to Kelly-Borges & Bergquist, Reference Kelly-Borges and Bergquist1997)
Encrusting sponges growing in circular to oblong patches, ~ 6 × 3 cm wide and 0.2 × 1 cm thick. Surface golden yellow to bright orange in life and cream in alcohol, with microscopically smooth, generally flattened triangular-shaped papillae, 3–15 mm long and 3–6 mm wide at base. Inhalant papillae separate from exhalant papillae, the latter with 2–3 wide exhalant canals and several narrower inhalant canals. Surface areas between the papillae obscured by silt and sand trapped by projecting spicules.
Skeleton
(Our observations)
Main choanosomal skeleton composed of longitudinal tracts (220–370 µm thick) of principal spicules which radiate in the cortex and terminate under a superficial palisade (Figure 6A). Auxiliary choanosomal skeleton comprises intermediary and small spicules scattered singly or arranged in randomly oriented groups, each of 3–5 spicules. These groups are accumulating in the base of the sponge, forming a layer along the substratum. Cortex made of two intermingled layers – a superficial palisade (180–270 µm thick) of bouquets of small tylostyles with single filiform subtylostyles interspersed in between and an inner layer (440–510 µm thick) of intermediary spicules (Figure 6B). Sparsely scattered exotyles cross the cortex with their distal extremities projecting above the surface. Papilla walls comprise the palisade of small tylostyles and a loose network of intermediary spicules.

Fig. 6. Proteleia tapetum, holotype NZNM Por 65: (A) longitudinal section through the body, general view; (B) the same section, detail of cortex; (C) principal strongyloxeas; (D) intermediary subtylostyles; (E) small tylostyles; (F) filiform styles; (G) exotyle, general view; (H) proximal tip of the exotyle depicted in G, detailed view; (I) distal ornamentation of the exotyle depicted in G, detailed view. Scale bars: A, 5 mm; B, 0.5 mm; C, 0.1 mm; D, 0.05 mm; E and F, 0.01 mm; G, 0.1 mm; H and I, 0.002 mm.
Spicules
(Our observations, N = 8 for exotyles and N = 10 for other categories)
-
• Principal spicules – strongyloxeas to fusiform subtylostyles, often polytylote (Figure 6C). Length 393–578–814 µm, proximal diameter of shaft 2.7–5.0–6.9 µm, maximum diameter of shaft 6.1–12.1–16.1 µm.
-
• Intermediary spicules – straight, occasionally curved, fusiform, often sabre-shaped subtylostyles (Figure 6D). Length 150–218–336 µm, diameter of tyle 5.3–6.2–8.1 µm, proximal diameter of shaft 3.9–4.6–6.0 µm, maximum diameter of shaft 6.6–8.5–11.8 µm.
-
• Small tylostyles gently curved, slender (Figure 6E). Length 74–85–98 µm, diameter of tyle 3.1–3.7–4.4 µm, diameter of shaft 2.4–2.8–3.2 µm.
-
• Filiform subtylostyles or styles extremely thin, considerably curved or bent (Figure 6F). Length 73–79–83 µm, diameter of shaft 0.8–1.2–1.6 µm.
-
• Exotyles gently curved, slender, 472–561–671 µm long, ~ 5 µm in diameter (Figure 6G). Their proximal extremities rounded, usually without tyles or more rarely with little swellings (Figure 6H). Distal ornamentations almost regular, umbrelliform to fungiform, with numerous short protuberances directed towards the proximal tips, 7.4–8.0–8.6 µm in width including the protuberances (Figure 6I).
REMARKS
Extremely thin exotyles with umbrelliform or fungiform distal ornamentations of Proteleia tapetum strongly resemble those of the type species of Proteleia, P. sollasi. The two species also exhibit very similar external morphology, both possessing a velvety surface with prominent papillae. However, the authors of P. tapetum (Kelly-Borges & Bergquist, Reference Kelly-Borges and Bergquist1997) considered these similarities as insufficient for the affiliation of their new species with Proteleia, emphasized the main difference between their species and P. sollasi (presence of an extra cortical palisade in the latter) and placed tapetum into Polymastia. At the same time the number and structure of cortical layers vary greatly among Polymastia spp. while the overwhelming majority of them including the type species P. mamillaris Müller, Reference Müller1806 lack ornamented exotyles. Hence we propose the assignment of tapetum to Proteleia.
Genus Sphaerotylus Topsent, Reference Topsent1898
TYPE SPECIES
Polymastia capitata Vosmaer, Reference Vosmaer1885 (by original designation).
DIAGNOSIS
Encrusting sponges of spherical, hemispherical, dome, cushion or button shape. Some species with a single papilla, others possess up to several tens of papillae. Main choanosomal skeleton made of radial or longitudinal tracts of principal monactines. These tracts ascend into the papillae. Auxiliary choanosomal skeleton comprises free-scattered, small and intermediary monactines, occasionally exotyles. A superficial cortical palisade composed of either exotyles with sparse small monactines or small monactines reinforced by exotyles. An inner layer of criss-cross intermediary monactines may be also present. Both cortical layers extend to the walls of prominent papillae. In less prominent papillae the walls are reinforced only by the palisade of small monactines. No exotyles present in the papillae. Small monactines are usually tylostyles. Intermediary and principal monactines vary from styles to tylostyles, the principal spicules often being polytylote. Distal extremities of exotyles rough, spined, granulated, tuberculated or wrinkled, often with knobs varying from spherical to hemispherical, fungiform, umbrelliform or lobate.
Sphaerotylus antarcticus Kirkpatrick, Reference Kirkpatrick1907
(Figures 7 & 8)

Fig. 7. Sphaerotylus antarcticus: (A) lectotype BMNH 1908.2.5.90, habitus; (B) specimen in situ in the Paradise Bay, Antarctic Peninsula (courtesy of N. Chervyakova, Moscow State University); (C) longitudinal section through the body of the lectotype, general view; (D) the same section, detail of cortex. Scale bars: A, 10 mm; C, 1 mm; D, 0.2 mm.

Fig. 8. Sphaerotylus antarcticus, spicules: (A) principal style; (B) longer intermediary subtylostyle; (C) shorter intermediary subtylostyle; (D) small spicules; (E) proximal tip of an exotyle, detailed view; (F) distal knob of the same exotyle, detailed view; (G) and (H) distal knobs of other exotyles, detailed view; (I) exotyles echinating the surface, view on a section. Scale bars: A, 0.3 mm; B, 0.1 mm; C and D, 0.03 mm; E–H, 0.01 mm; I, 0.2 mm.
Original description: Sphaerotylus antarcticus Kirkpatrick, Reference Kirkpatrick1907, p. 272.
SYNONYMS AND CITATIONS
Sphaerotylus antarcticus (Kirkpatrick, Reference Kirkpatrick1908, p. 16, pl. XII figures 1a–16 and pl. XIII figures 1–7; Burton, Reference Burton1929, p. 446, Reference Burton1932, p. 339; Koltun, Reference Koltun, Pavlovskii, Andriyashev and Ushakov1964, p. 27, pl. V figures 14–20; Vacelet & Arnaud, Reference Vacelet and Arnaud1972, p. 14; Desqueyroux-Faúndez, Reference Desqueyroux-Faúndez1989, p. 107; Barthel et al., Reference Barthel, Tendal and Panzer1990, p. 122).
Sphaerotylus borealis antarcticus (Koltun, Reference Koltun1976, p. 168; Sarà et al., Reference Sarà, Balduzzi, Barbieri, Bavestrello and Burlando1992, p. 568).
TYPE MATERIAL
Lectotype (designated herein, see Figure 7A, specimen preserved in alcohol and depicted by Kirkpatrick (Reference Kirkpatrick1908) in pl. XII, figure 1A): BMNH 1908.2.5.90, Flagon point of Winter Quarters, Winter Quarters Bay, McMurdo Sound, Ross Sea, Southern Ocean, 77°50′42.77″S 166°39′1.41″E, 18–36.5 m (10–20 fathoms), British National Antarctic Expedition on RV ‘Discovery’ in 1901–1904, 21.01.1903.
Paralectotypes: BMNH 1908.2.5.91–96 and 1908.2.5.99–99A (10 specimens in alcohol), BMNH 1908.2.3.109 (one dry specimen), BMNH 1908.2.3.100–108 (23 slides prepared from the type series), BMNH 1908.2.5.97, 98 and 110 (specimens considered lost), Winter Quarters Bay, McMurdo Sound, Ross Sea, Southern Ocean, 77°50′42.77″S 166°39′1.41″E, 18–54.5 m (10–30 fathoms), British National Antarctic Expedition on RV ‘Discovery’ in 1901–1904.
COMPARATIVE MATERIAL EXAMINED
USNM (no number), NW side of New Rock, vicinities of the Palmer US research station, Antarctic Peninsula, Bellingshausen Sea, Southern Ocean, 12.2 m, scuba diving survey, station 103H74, 12.01.1974 (six specimens). USNM (no number), Cape Bellue, vicinities of the Palmer US research station, Antarctic Peninsula, Bellingshausen Sea, Southern Ocean, 66°18′S 65°53′W, 13.7 m, scuba diving survey, station 299H74 (one specimen). ZMBN 98045, Almirante Brown Antarctic Base, Paradise Bay, Bellingshausen Sea, Southern Ocean, 64°54.4′S 62°52.0′W, 21 m, 06.03.2010, coll. N. Chervyakova (one specimen). ZIN RAS (no number), ‘Molodezhnaya’ Russian research station, Cosmonaut Sea, Southern Ocean, 67°40.3′S 45°23′E, 3 m, The 11th Soviet Antarctic Expedition, scuba diving survey, transect II, station 3, 06.03.1966, coll. Propp (three specimens).
DESCRIPTION
External morphology
Lectotype (Figure 7A) thickly encrusting, 8 × 8 × 2.5 cm in size, overgrowing a volcanic concretion together with the specimen BMNH 1908.2.5.75 (syntype of Polymastia invaginata). Surface shaggy, dirty grey, with 15 light-coloured papillae. Most papillae well-defined, conical, 0.9–2.5 cm long, 0.3–1 mm in diameter at base, bearing oscula on the tops. Some papillae damaged. One of these sectioned transversally demonstrating a wide central canal with several narrow peripheral canals. Three papillae considerably contracted. Paralectotypes vary greatly in shape, size and prominence of papillae. Larger sponges usually flattened, encrusting. Smaller sponges may be dome-shaped or subspherical. In the smallest specimens the length of papilla may exceed the body dimensions by up to three times. Other studied sponges thickly encrusting or cushion-shaped, the largest specimens up to 200 cm2. Surface shaggy and heavily dusted with sediment making it dirty greyish or brownish. In life the sponges are often covered by sediment with erect papillae protruding above the sediment (Figure 7B). After sampling and fixation the papillae contract and invaginate into the surface hispidation. Sponges may have up to 50 papillae which are usually slender and cylindrical, more rarely stout and conical, with oscula visible on their summits, colouration yellowish in life and more pale in alcohol.
Skeleton
Main choanosomal skeleton composed of radial or longitudinal tracts of principal spicules crossing the cortex and making up a dense and thick surface hispidation (Figure 7C). Auxiliary choanosomal skeleton comprises singly scattered small, occasionally intermediary, spicules. Cortical palisade (165–170 µm thick) of small spicules (Figure 7D), lying directly on a layer (700–800 µm thick) of tangentially arranged intermediary spicules. Exotyles cross the cortex and join the superficial hispidation (Figure 8I).
Spicules
(measurements based on five specimens, N = 5 for exotyles, N = 10 for other categories):
-
• Principal spicules – straight, slender, often polytylote subtylostyles to styles (Figure 8A). Length 900–1870–2900 µm, proximal diameter of shaft 17.0–19.5–23.0 µm, maximum diameter of shaft 20.0–32.3–41.0 µm.
-
• Intermediary spicules – straight, stout subtylostyles to tylostyles (Figure 8B, C). Length 240–490–630 µm, diameter of tyle 8.0–14.8–20.0 µm, proximal diameter of shaft 7.0–9.0–10.0 µm, maximum diameter of shaft 10.0–14.2–20.0 µm.
-
• Small spicules – straight or gently curved, strongly fusiform, sabre-shaped tylostyles to subtylostyles (Figure 8D). Length 100–123–150 µm, diameter of tyle 3.0–3.2–3.5 µm, proximal diameter of shaft 2.5–2.6–3.0 µm, maximum diameter of shaft 5.5–6.2–7.0 µm.
-
• Exotyles slender, 1000–4656–8000 µm long, shaft diameter 20.0–23.6–30.0 µm. Proximal tyles usually weakly developed or absent (Figure 8E). Distal knobs 24.0–29.9–40.0 µm in diameter, irregular, varying from subspherical to hemispherical, fungiform or umbrelliform, occasionally with short protuberances on the edges (Figure 8F–H). Surface of the knobs and the adjacent portions of the shaft rough, granulated, tuberculated or wrinkled.
OCCURRENCE
(Figure 3)
Southern Ocean: continental sectors 2, 3 (Davis Sea), 4 (Adélie Land), 5 (Ross Sea), 8 (Bellingshausen Sea, Antarctic Peninsula), 9 (Weddell Sea) (sectors numbered according to Sarà et al., Reference Sarà, Balduzzi, Barbieri, Bavestrello and Burlando1992), 3–437 m, South Shetland Islands, 20–60 m (data by Desqueyroux-Faúndez, Reference Desqueyroux-Faúndez1989).
REMARKS
Sphaerotylus antarcticus is very similar to S. borealis from the northern hemisphere. Both species are characterized by a shaggy surface, two-layered cortex and extremely long exotyles with irregular distal knobs varying from subspherical to fungiform and umbrelliform, the features distinguishing them from the type species of Sphaerotylus, S. capitatus (Vosmaer, Reference Vosmaer1885). The similarities between S. antarcticus and S. borealis led Koltun (Reference Koltun1976) to the assumption that they were subspecies of a single species with a bipolar distribution. The only obvious difference between these two is the sabre-like shape of the small tylostyles in S. antarcticus. The shaggy surface and extremely long exotyles like in S. antarcticus and S. borealis are also recorded in Koltunia burtoni. However, the latter species is distinguished by the cortex lacking the ordinary superficial palisade and the inner spicule layer, and by the unique shape of its exotyles bearing huge grapnel-like ornamentations on the distal extremities.
Sphaerotylus antarcticus drygalskii Hentschel, Reference Hentschel and von Drygalski1914
(Figure 9)

Fig. 9. Sphaerotylus antarcticus drygalskii: (A) lectotype ZMB 4836, habitus; (B) paralectotype ZMB 4836, habitus; (C) and (D) longitudinal sections through the body of the type specimens; (E) small tylostyle; (F) and (G) distal knobs of exotyles, detailed view. Scale bars: A and B, 1 mm; C and D, 0.5 mm; E–G, 0.02 mm.
Original description: Sphaerotylus antarcticus var. drygalskii Hentschel, Reference Hentschel and von Drygalski1914, p. 51.
TYPE MATERIAL
Lectotype (designated herein, see Figure 9A): ZMB 4836 (specimen in alcohol), Gauss-Station, Davis Sea, Southern Ocean, 66°02′S 89°38′E, 385 m, Deutschen Südpolar-Expedition, 17.12.1902.
Paralectotype (Figure 9B): ZMB 4836 (one specimen in alcohol), from the same sample as the holotype.
Paralectotype (considered lost): ZMB 4836, the same expedition and locality as for the holotype, 380 m, 22.01.1903.
DESCRIPTION
External morphology
Both lectotype and paralectotype cushion-shaped. Lectotype 0.8 × 0.6 × 0.2 cm in size, detached from substratum (Figure 9A). Paralectotype 0.4 × 0.4 × 0.1 cm in size, attached to a piece of dead bryozoan skeleton (Figure 9B). Surface of both sponges strongly hispid and heavily dusted with sediment making it dirty greyish in colour. Each sponge with a prominent, almost regularly cylindrical central papilla (~ 0.5 cm long in the lectotype and 0.1 cm long in the paralectotype) and few contracted and damaged pin-like peripheral papillae. Oscula not visible.
Skeleton
Main choanosomal skeleton composed of radial or longitudinal tracts of principal spicules which cross the cortex and make up a dense surface hispidation (Figure 9C, D). Auxiliary choanosomal skeleton comprises singly scattered small, occasionally intermediary, spicules. In cortex a palisade (~ 140 µm thick) of small spicules is intermingled with an internal layer (~170 µm thick) of tangentially arranged intermediary spicules. Exotyles cross the cortex and join the superficial hispidation.
Spicules
(measurements based on lectotype and paralectotype, N = 5 for exotyles, N = 10 for other categories)
-
• Principal spicules – straight, slender, occasionally polytylote subtylostyles to styles. Length 600–723–900 µm, diameter of shaft 10.0–10.4–11.0 µm.
-
• Intermediary spicules – gently curved or straight subtylostyles to tylostyles. Length 365–440–520 µm, diameter of the shaft 8.0–9.2–10 µm.
-
• Small spicules – straight or gently curved, slightly fusiform tylostyles (Figure 9E). Length 100–117–132 µm, diameter of shaft 5.0–5.6–6.0 µm.
-
• Exotyles slender, 750–817–900 µm long, shaft 9.0–10.1–11.0 µm in diameter. Proximal tyles usually weakly developed or absent. Distal knobs 18.0–19.6–21.0 µm in diameter, often regularly fungiform, occasionally subhemispherical, always with granulated surface (Figure 9F, G).
OCCURRENCE
(Figure 3)
Known only from the type locality near Gauss Station, Davis Sea, Southern Ocean.
REMARKS
The only apparent difference between Sphaerotylus antarcticus drygalskii and typical S. antarcticus is that all three categories of spicules are shorter in the former.
Sphaerotylus borealis (Swarczewsky, Reference Swarczewsky1906)
(Figures 19 & 20)
Original description: Proteleia borealis Swarczewsky, Reference Swarczewsky1906, p. 315, pl. X figure 1, pl. XIII figure 2.
SYNONYMS AND CITATIONS
Proteleia borealis (Boury-Esnault, Reference Boury-Esnault, Hooper and van Soest2002, p. 204).
Sphaerotylus borealis (Rezvoj, Reference Rezvoj1928, p. 78, figures 4 & 5; Koltun, Reference Koltun1966, p. 83, pl. XXX figures 1 & 5, text-figure 55; Plotkin, Reference Plotkin, Pansini, Pronzato, Bavestrello and Manconi2004, p. 543, figures 1I, 2I, 4B).
Sphaerotylus schoenus var. borealis (Hentschel, Reference Hentschel, Römer, Schaudinn, Brauer and Arndt1929, p. 925).
TYPE MATERIAL
Holotype (small fragment, considered lost): Small Pir'yu Inlet, near Umba, Kandalaksha Bay, White Sea, ~ 66°40.37′N 34°19.7′E, 5.5 m, coll. Varpakhovsky.
Neotype (designated herein, see Figure 10A): ZIN RAS 11194 (specimen in alcohol), Sredny Island, Keret’ Inlet, Kandalaksha Bay, White Sea, 66°17.391′N 33°38.025′E, 10–13 m, 12.07.2000, coll. Plotkin.

Fig. 10. Sphaerotylus borealis: (A) neotype ZIN RAS 1194, habitus; (B) longitudinal section through the body of a White Sea specimen; (C) longitudinal section through a papilla of the White Sea specimen. Scale bars: A, 10 mm; B and C, 0.3 mm.
COMPARATIVE MATERIAL EXAMINED
Arctic Ocean (one specimen):
ZIN RAS 11178 (one specimen, slides 6084, 6082, 7136–7141), between Svalbard and Franz Josef Land, 82°00′N 42°00′E, 415 m, RV ‘Litke’, station 26, 18.09.1955, coll. Koltun.
Barents Sea (21 specimens):
ZIN RAS 11145 (one specimen), 72°30′N 23°01′E, 342 m, RV ‘Dalnie Zelentsy’, cruise 16, station 25, 05.10.1982. ZIN RAS 11146 (one specimen), 73°00′N 35°14′E, 219 m, RV ‘Dalnie Zelentsy’, cruise 24, station 14, 22.08.1984. ZIN RAS 11156 (one specimen, slide 5527), 73°02′N 25°58′E, 420 m, Expedition of PMNI, station 660, 12.06.1927. ZIN RAS 11157 (one specimen, slide 7882), 75°38′N 30°00′E, 331 m, Expedition of PMNI, station 966, 22.06.1928. ZIN RAS 11158 (one specimen, slide 5523), 72°00′N 35°00′E, 256 m, Expedition of PMNI, station 1062, 17-18.08.1928. ZIN RAS 11159 (one specimen, slide 7884), 70°55′N 37°33′E, 249 m, Expedition of PMNI, station 631, 29.05.1927. ZIN RAS 11160 (one specimen), 69°35′N 33°40′E, 180 m, Expedition of PINRO, RV ‘Persey’, cruise 53, station 3064, 10.05.1935. ZIN RAS 11163 (one specimen), 70°39′N 33°30′E, 243 m, Expedition of ENPIM, RV ‘St. Andrew Pervozvanny’, station 467, 16(29).05.1900, coll. Breitfuss. ZIN RAS 11166 (one specimen), 70°45′N 33°30′E, 260 m, RV ‘Maslov’, cruise 1, station 7/183, 29.11.1968. ZIN RAS 11167 (one specimen), 72°30′N 33°30′E, 142 m, trawl 15, sample 12, 29.05.1924, coll. Ushakov. ZIN RAS 11170 (one specimen), 69°26.5′N 36°34′E, 200 m, RV ‘Prof. Derugin’, cruise 8, station 155, 09.10.1959, coll. Galkin. ZIN RAS 11171 (one specimen), 69°00′N 38°00′E, 175 m, RV ‘RT61-Vodnik’, cruise 26, station 105, 10.07.1968. ZIN RAS 11174 (one specimen, slide 13403), 69°23.1′N 34°29′E, 130 m, Expedition of Murmansk Biological station, RV ‘Diana’, station 27, 25.09.1953. ZIN RAS 11176 (one specimen, slide 13597), 69°20′1″N 35°12′8″E, 153 m, Expedition of Murmansk Biological station, station 37, 29.03.1954. ZIN RAS 11177 (one specimen, slides 13309, 13311), 69°11.4′N 36°11′E, 170–165 m, RV ‘Prof. Derugin’, cruise 8, station 153, 10.10.1958, coll. Galkin. ZIN RAS 11181 (one specimen), 71°00′N 35°40′E, 215 m, Expedition of Murmansk Biological station, station 117a, 28.06.1958, coll. Vilenkin. ZIN RAS 11183 (one specimen, slide 13428), 69°01′N 36°41′E, 128 m, Expedition of Murmansk Biological station, RV ‘Diana’, station x-1, 14.07.1955. ZIN RAS 11168 (one specimen, slide 5519), Gavrilovo, near the entrance to the bight, Murman Coast, 69°10′56.88″N 35°51′10.45″E, 91 m, station 154/72, 02.08.1894, coll. Knipovich. ZIN RAS 11164 (one specimen, slide 5511), Kildin Straight, Murman Coast, 69°18′49.02″N 34°07′17.13″E R/V ‘Alexander Kovalevsky’, cruise 43, 31.07.1924, coll. Derugin. ZIN RAS 11173 (one specimen, slide 9131), Kola Bay, Murman Coast RV ‘Alexander Kovalevsky’, 1908–1909, coll. Derugin. ZIN RAS 11165 (one specimen, slide 0095), Rybachy Peninsula, Murman Coast, 69°55′N 32°38.75′E, 124 m, Expedition of ENPIM, RV ‘St. Andrew Pervozvanny’, station 716, 04(17).08.1900, coll. Breitfuss.
Between Kara and Laptev Sea (one specimen):
ZIN RAS 11179 (one specimen, slides 5524, 12299), Shokalsky Straight, 78°48.3′N 99°26′E, 43 m, RV ‘Rusanov’, station 9 (iii, i), 19.08.1932, coll. Vagin & Kondakov.
Norwegian Sea (two specimens):
ZIN RAS 11169 (one specimen, slide 8614), 64°45.8′N 12°31.1′E, 157 m, RV ‘Sebastopol’, cruise 8, station 1427, 09.04.1958, coll. Zatsepin. ZIN RAS 11184 (one specimen, slide 10258), 66°52′N 14°E, 240 m, RV ‘SRT4225′, cruise 1, station 61/127, 21.06.1955, coll. Kobyakova.
White Sea (31 specimens):
ZIN RAS 11148 (one specimen), Basin of the White Sea, 66°08′N 37°31.3′E, 24–31 m, RV ‘Pomor’, station 20(36), 30.05.1983, coll. Gudimov. ZIN RAS 11149 (one specimen), Dvina Bay, 65°10′N 37°10′E, 37 m, RV ‘Pomor’, station 11, 29.05.1983, coll. Gudimov. ZIN RAS 11144 (one specimen), near White Sea Biological Station of ZIN RAS, Chupa Inlet, Kandalaksha Bay, 19–22 m, station, 20.10.1967, coll. Golikov. ZIN RAS 11151 (one specimen, slide 21068), Chupa Inlet, Kandalaksha Bay, 66°18.3′N 33°49.5′E, 20 m, RV ‘Onega’, station 17/361, 19.07.1964, coll. Kunin. ZIN RAS 11152 (one specimen, slide 21069), Chupa Inlet, Kandalaksha Bay, 21–26 m, RV ‘Onega’, station 33/15, 21.07.1961, coll. Kunin. ZIN RAS 11153 (one specimen, slide 21070), Chupa Inlet, Malaya Klyuschikha Bight, Kandalaksha Bay, 5–20 m, RV ‘Onega’, station 5/347, 10.07.1964, coll. Kunin. ZIN RAS 11180 (one specimen), Chupa Inlet, Levaya Bight, Kandalaksha Bay, 20 m, station 9, transect 3, square 0.1 m2, 21.07.1977, coll. Golikov. ZIN RAS 11194 (one specimen), Keret’ Inlet, Sredny Island, Nagovitsa Harbour, Black Rock, Kandalaksha Bay, 66°17.391′N 33°38.025′E, 10–13 m, station, 12.07.2000, coll. Plotkin. ZIN RAS 11195 (16 specimens), Keret’ Inlet, Sredny Island, Nagovitsa Harbour, Black Rock, Kandalaksha Bay, 66°17.391′N 33°38.025′E, 10–13 m, station, 12.07.2000, coll. Plotkin. ZIN RAS 11150 (one specimen, slide 21064), Kolvitsa Inlet, Kandalaksha Bay, 67°05.1′N 32°54.4′E, 20–30 m, RV ‘Prof. Mesyatsev’, station 856/5, 27.10.1961, coll. Kunin. ZIN RAS 11161 (one specimen, slide 5874), Kovda Inlet, Startseva Bight, Kandalaksha Bay Expedition of Voronezh University, 27.06.1917, coll. Sent-Iler. ZIN RAS 11162 (one specimen, slide 5609), Kovda Inlet, between Oleniy Island and Medvezhiy Island, Kandalaksha Bay, 10–12 m, Expedition of Voronezh University, 1917 or 1921, coll. Sent-Iler. ZIN RAS 11182 (one specimen, slide 9138), Umba Inlet, Kandalaksha Bay, 32 m, station 31(195), 27.06.1895, coll. Knipovich. ZIN RAS 11147 (one specimen), Neck of the White Sea, 65°45′N 39°00′E, 57 m, RV ‘Pomor’, station 51(15), 02.06.1983, coll. Gudimov. ZIN RAS 11155 (one specimen, slide 5525), Neck of the White Sea, 65°36′N 39°25′E, 54 m, Expedition of PMNI, station 57, 26.09.1921. ZIN RAS 11175 (one specimen, slide 9123), Onega Bay, 64°44′N 35°42.5′E, 30 m, Expedition of PMNI, station 448, 09.06.1926.
DESCRIPTION
External morphology
Holotype was a 3 × 1.5 × 1 cm piece torn from a large encrusting sponge during sampling. Surface was shaggy, with several whitish cylindrical or conical papillae up to 1 cm in length, some with visible oscula on the summits (description according to Swarczewsky, Reference Swarczewsky1906). Neotype is a flattened thickly encrusting sponge measuring 4.5 × 2 × 1 cm (Figure 10A). Surface shaggy, dirty dark brown, overgrown with two ascidians. Twelve cylindrical yellowish papillae up to 0.7 cm long and 0.2 cm wide. Other specimens thickly encrusting or cushion-shaped, the largest up to 100 cm2. Surface shaggy, silted with sediment making it dirty greyish or brownish in colour. Up to 50 cylindrical or conical papillae, whitish in life, but usually becoming pale yellow, brownish or pinkish in alcohol. On soft bottoms living sponges are often completely covered by sediment with only erect papillae protruding above the sediment. On hard bottoms the sponges may contract the papillae. After sampling and fixation the papillae always considerably contract and invaginate into the surface hispidation. Oscula not visible in preserved sponges.
Skeleton
Main choanosomal skeleton composed of longitudinal tracts of principal spicules which cross the cortex and make up a dense and thick surface hispidation (Figure 10B). Auxiliary choanosomal skeleton comprises small, occasionally intermediary, spicules often arranged in bundles, 3–7 spicules each. Cortex composed of a 115–120 µm thick palisade of small spicules and an internal layer (~ 210 µm thick) of tangentially arranged intermediary spicules (Figure 10B). In areas around papillae these layers are separated by an intermediate, aspicular zone (~ 100 µm thick) (Figure 19B). Exotyles cross the cortex and join the surface hispidation. Walls of papillae lack the tangential cortical layer. Single intermediary spicules scattered both in the walls and in the bulkheads between canals (Figure 10C).
Spicules
(measurements based on 10 specimens, N = 5 for exotyles, N = 10 for other categories)
-
• Principal spicules – straight, slender, often polytylote styles to subtylostyles (Figure 11A–F). Length 1100–2423–5000 µm, diameter of shaft 12.0–16.2–19.0 µm.

Fig. 11. Sphaerotylus borealis, spicules: (A) principal style, general view; (B) proximal tip of the style depicted in A, detailed view; (C) distal tip of the style depicted in A, detailed view; (D) principal subtylostyle, general view; (E) proximal tip of the subtylostyle depicted in D, detailed view; (F) distal tip of the subtylostyle depicted in D, detailed view; (G) intermediary tylostyles; (H) small tylostyles; (I) distal ornamentations of exotyles, detailed view. Scale bars: A, 0.1 mm; B and C, 0.01 mm; D, 0.1 mm; E and F, 0.01 mm; G, 0.1 mm; H and I, 0.01 mm.
-
• Intermediary spicules – usually straight, occasionally curved, slightly fusiform tylostyles (Figure 11G). Length 200–502–796 µm, diameter of tyle 6.9–9.2–11.1 µm, proximal diameter of shaft 5.0–7.1–9.0 µm, maximum diameter of shaft 6.9–10.8–14.3 µm.
-
• Small spicules – straight or curved, usually slender tylostyles (Figure 11H). Length 94–125–160 µm, diameter of tyle 3.9–4.6–5.1 µm, diameter of shaft 3.0–3.5–4.0 µm.
-
• Exotyles slender, 5100–6117–7520 µm long, usually with weakly developed or completely reduced proximal tyles. Shafts 13.8–17.2–20 µm in maximum diameter. Distal knobs (14.1–19.9–27.0 µm in diameter) usually irregularly fungiform or umbrelliform, more rarely hemispherical or spherical, occasionally with short protuberances on the edges, sometimes slightly displaced along the shaft or comprising several swellings (Figure 11I). Surface of the knobs and the adjacent portions of the shafts rough, wrinkled, granulated or tuberculated.
-
• In their material, Swarczewsky (Reference Swarczewsky1906) and Koltun (Reference Koltun1966) recorded infrequent thick and short fusiform strongyles (length 464–1300 µm, maximum diameter 40–59 µm) in the cortex, but in the sponges examined in the present study this category of spicules has not been observed.
OCCURRENCE

Fig. 12. Distribution of Polymastiidae with ornamented exotyles in the North Atlantic and Arctic: stars, Sphaerotylus borealis; circles, Sphaerotylus capitatus; triangles, Sphaerotylus renoufi; square, Sphaerotylus tjalfei; cross, Trachyteleia stephensi; heart, Tylexocladus joubini.
Arctic Ocean: between Svalbard and Franz Jozef Land, 415 m, between Kara and Laptev Sea, 43 m, Barents Sea, 91–420 m, White Sea, 5–100 m. North Atlantic: Norwegian Coast – Nord-Trøndelag, 157–240 m.
REMARKS
Sphaerotylus borealis (Swarczewsky, Reference Swarczewsky1906) was originally assigned to Proteleia Dendy & Ridley, Reference Dendy and Ridley1886, due to the similarity between the umbrelliform distal knobs of some exotyles in S. borealis and the grapnel-like distal ornamentations of the exotyles in the type species of Proteleia, P. sollasi. This placement was later followed by Boury-Esnault (Reference Boury-Esnault, Hooper and van Soest2002). However, P. sollasi differs from S. borealis by a velvety surface, a three-layered cortex comprising two palisade layers and an inner layer of criss-cross spicules, and much shorter exotyles (not exceeding 0.6 mm). Substantial affinities between Sphaerotylus borealis and S. antarcticus along with their differences from the type species of Sphaerotylus, S. capitatus, and their similarities to Koltunia burtoni are discussed above in the Remarks section for S. antarcticus.
Sphaerotylus capitatus (Vosmaer, Reference Vosmaer1885)
(Figures 13 & 14)

Fig. 13. Sphaerotylus capitatus: (A) lectotype RMNH 704, habitus; (B) paralectotype RMNH 704, habitus; (C) specimen ZMBN 98075 in situ near Haugbergnes, Troms, Norwegian Sea (courtesy of B.T. Dragnes, OMNIMAR Dragnes, Tromsø); (D) longitudinal section through the body of the lectotype, general view. E, the same section, detail of cortex; (F) the same section, detail of choanosome with exotyles; (G) longitudinal section through a papilla of a specimen from Hordaland, Norway. Scale bars: A and B, 10 mm; D, 1 mm; E, 0.2 mm; F and G, 0.2 mm.

Fig. 14. Sphaerotylus capitatus, spicules: (A) principal subtylostyle; (B) intermediary tylostyle; (C) small tylostyles; (D) exotyle, general view; (E) proximal tip of the exotyle depicted in D, detailed view; (F) distal knob of the exotyle depicted in D, detailed view. Scale bars: A–D, 0.1 mm; E and F, 0.01 mm.
Original description: Polymastia capitata Vosmaer, Reference Vosmaer1885, p. 16, pl. IV figures 25–29.
SYNONYMS AND CITATIONS
Polymastia capitata (Breitfuss, Reference Breitfuss1911, p. 218).
Polymastia schoenus (Dendy & Ridley, Reference Dendy and Ridley1886, p. 155, text-fig.).
Radiella schoenus (Sollas, Reference Sollas1882, p. 162, considered as nomen nudum by Kirkpatrick, Reference Kirkpatrick1908, p. 18).
Sphaerotylus capitatus (Topsent, Reference Topsent1898, p. 244; Boury-Esnault, Reference Boury-Esnault, Hooper and van Soest2002, p. 206, figure 4; Plotkin, Reference Plotkin, Pansini, Pronzato, Bavestrello and Manconi2004, p. 543, figures 1H, 2H, 4A).
Sphaerotylus schoenus (Topsent, Reference Topsent1913, p. 23, pl. II figure 6; Reference Topsent1928, p. 154; Koltun, Reference Koltun1966, p. 85, pl. XXX figures 6 & 7, text-figure 56; Desqueyroux-Faúndez & Van Soest, Reference Desqueyroux-Faúndez and van Soest1997, p. 421).
Nec Sphaerotylus capitatus (Kirkpatrick, Reference Kirkpatrick1908, p. 18; Boury-Esnault & Van Beveren, Reference Boury-Esnault and van Beveren1982, p. 39; Uriz, Reference Uriz1988, p. 43; Sarà et al., Reference Sarà, Balduzzi, Barbieri, Bavestrello and Burlando1992, p. 568).
Nec Sphaerotylus schoenus (Burton, Reference Burton1929, p. 447; Koltun, Reference Koltun, Pavlovskii, Andriyashev and Ushakov1964, p. 28; Sarà et al., Reference Sarà, Balduzzi, Barbieri, Bavestrello and Burlando1992, p. 568).
TYPE MATERIAL
Lectotype (Figure 13A, specimen in alcohol) and one paralectotype (specimen in alcohol) (Figure 13B): RMNH 704, Barents Sea, 72°14.8′N 22°30.9′E, ~ 300 m (165 fathoms), ‘Willem Barentz’ Expedition, station 28, 30.06.1881.
Paralectotype: BMNH 1910.1.1.612 (specimen in alcohol) and BMNH 1910.1.1.1196-1200 (slides), from the same sample as the lectotype.
Paralectotype: ZMA 1841 (specimen, not studied), from the same sample as the lectotype.
COMPARATIVE MATERIAL EXAMINED
Barents Sea (six specimens):
ZIN RAS 1186 (slide 5445), at the traverse of Bolshaya Voronukha Island, Kola Bay, Murman Coast, 69°16′31.43″N 33°27′23.31″E, 213–235 m, RV ‘Alexander Kovalevsky’, station 93, 26.06.1909, coll. Derugin (one specimen). ZIN RAS 1187 (slide 5573), Cape Teriberka, Murman Coast, 69°15′08.45″N 35°09′03.95″E, depth unknown, 1880, coll. Hertzenstein (one specimen). ZIN RAS 1188 (slide 5957), near the exit from the Kola Bay to the Ekaterininskaya Harbour, Murman Coast, 69°12′33.96″N 33°26′52.23″E, 55–31 m, station 21, 21.06.1893, coll. Knipovich (one specimen). ZIN RAS 1189, 75°42′N 47°05′E, 309 m, expedition of ENPIM, RV ‘St. Andrew Pervozvanny’, station 705, 13.08.1902 (one specimen). ZIN RAS 1190, 71°30′N 25°30′E, 275 m, RV ‘RT61-Vodnik’, cruise 25, station 39, 10.06.1968 (one specimen). ZIN RAS 1191 (slides 7550–7551), 69°43′N 34°10′E, 142 m, Expedition of PMNI, station 295, 10.07.1925 (one specimen).
Svalbard (two specimens):
ZIN RAS 1185 (slides 6058, 12298, 12300), North from Svalbard, 80°35′N 13°35′E, 819 m, RV ‘Litke’, station 49, 11.10.1955, coll. Koltun (one specimen). ZIN RAS 1192 (slide 6844), SW from Svalbard, precise locality unknown, 608 m, RV ‘Lena’, station 1a, 11.03.1958, coll. Gorunova & Petrovskaya (one specimen).
Greenland (one specimen):
ZIN RAS 1193 (slide 14714), East Greenland, 64°13′N 38°48′W, 420–450 m, RV ‘RT 97′, cruise 21, 1964.
Norwegian Coast (six specimens):
ZMBN 98042, Hordaland, Korsfjorden, North of Stora Skorpa, 60°09.702′ N 5°10.4832′ E, 500–200 m, 10.03.2006, coll. Rapp (one specimen). ZMB 10855, Hordaland, Byfjorden near Bergen, depth unknown, coll. Schaudinn, 1891 (one specimen, misidentified as Polymastia uberrima (Schmidt, Reference Schmidt1870) by Arndt). HTR, Hordaland, Bømlafjorden, SE from Store Bleikja, 59°36.700–36.750′N 05°15.785–15.450′E, 300–78 m, RV ‘Hans Brattstrøm’, station 13, 04.07.2006, coll. Rapp (one specimen). HTR, Møre & Romsdal, 62°43.81′N 06°57.80′E, depth unknown, RV ‘Håkon Mosby’, station 33(329), 12.10.2005, coll. Rapp (one specimen). HTR, Møre & Romsdal, 62°54.12′N 06°50.53′E, 130–190 m, RV ‘Håkon Mosby’, station 38, 12.10.2005, coll. Rapp (one specimen). ZMBN 98075, Tromsø, Haugbergnes, 69°31.16′ N 19°00.68′ E, 25 m, 20.06.2012, coll. Plotkin (one specimen).
Swedish Coast (four specimens):
GNM 899, 58°28.357–28.308′N 10°29.646–29.289′E, 239–314 m, Expedition of the Swedish marine inventories, station SK 119, 29.08.2007, coll. Hansson (one specimen). GNM 900, 58°26.336–26.447′N 10°31.041–30.852′E, 265–309 m, Expedition of the Swedish marine inventories, station SK 121, 29.08.2007, coll. Hansson, (two specimens). GNM 902, 58°24.530–24.678′N 10°29.877–29.537′E, 266–317 m, Expedition of the Swedish marine inventories, station SK 159, 14.06.2008, coll. Hansson (one specimen).
DESCRIPTION
External morphology
Lectotype fist-shaped sponge, 2–2.5 cm in diameter, attached to a stone and incorporating a piece of a hard coral skeleton (Figure 13A). Surface rough, knobbly and brownish. Several weakly developed or contracted pale papillae. Paralectotype RMNH 704 dome-shaped, 1.4 cm high (Figure 13B). Surface slightly hispid, with a single well-developed but invaginated papilla. Other sponges thickly encrusting, cushion-shaped or massive, fist- and dome-shaped, the largest up to 50 cm2. Surface velvety, knobbly, dark brown in colour, with up to 30 papillae. Papillae of living sponges whitish or pale yellow in colour, conical, with small scarcely visible oscules on the summits (Figure 13C). In alcohol-preserved specimens the papillae may be considerably contracted looking like tubercles, while their colour does not change much.
Skeleton
Main choanosomal skeleton composed of radial or longitudinal tracts of principal spicules which enter the cortex (Figure 13D, E). Auxiliary choanosomal skeleton comprises small and intermediary spicules usually scattered singly or sometimes arranged in small groups. Some specimens including the lectotype and paralectotype BMNH 10.1.1.1199–1200 also possess exotyles between the choanosomal tracts (Figure 13F). Cortex composed of an outer palisade (~ 110 µm thick) of small spicules, an inner layer (~ 170 µm thick) of tangentially arranged intermediary spicules and an intermediate layer (180–190 µm thick) with a low concentration of spicules. Exotyles cross the cortex forming a dense superficial layer with their distal knobs rising above the palisade (Figure 13E). Papillae walls without the inner cortical layer (Figure 13G). Single intermediary spicules scattered both in the papillae walls and in the bulkheads between the canals.
Spicules
(measurements based on five specimens, N = 10)
-
• Principal spicules – straight, slightly fusiform or slender, often polytylote subtylostyles to styles (Figure 14A). Length 650–998–1505 µm, diameter of tyle if present 10.0–12.8–16.0 µm, proximal diameter of shaft 8.9–11.5–15.1 µm, maximum diameter of shaft 14.0–19.5–26.0 µm.
-
• Intermediary spicules – straight or gently curved, slender or slightly fusiform tylostyles (Figure 14B). Length 314–484–650 µm, diameter of tyle 9.1–11.4–14.0 µm, proximal diameter of shaft 6.9–8.8–11.0 µm, maximum diameter of shaft 9.0–13.0–16.5 µm.
-
• Small spicules – straight or curved, usually slender tylostyles (Figure 14C). Length 96–155–221 µm, diameter of tyle 2.9–4.6–6.1 µm, proximal diameter of shaft 1.1–2.3–3.2 µm, maximum diameter of shaft 2.0–5.0–7.0 µm.
-
• Exotyles straight or gently curved, slender, 650–974–1250 µm long (Figure 14D). Proximal tyles varying from well-developed (6.8–11.0–14.0 µm in diameter) to reduced (Figure 14E). Distal knobs usually regularly spherical, occasionally hemispherical or elongated, 18.0–22.8–30.0 µm in diameter. Surface of the knobs and the adjacent portions of the shafts usually rough, spined or granulated (Figure 14F). Shafts gradually expanding towards the distal knobs.
OCCURRENCE
Arctic Ocean: Barents Sea, 31–309 m, North Svalbard, 608–819 m. North Atlantic: Norwegian Coast – from Troms in the north to Sunnhordland in the south, 25–440 m, Swedish Western Coast, 239–317 m, East Greenland, 420–450 m, Canadian Coast – Nova Scotia, 75 m (data from Topsent, Reference Topsent1928).
REMARKS
This well-defined and widely known North Atlantic species has a confused synonymy. In 1882 Sollas mentioned very briefly his new species Radiella schoenus when discussing the characters of Tetilla and Rhaphidotheca: ‘The rounded swelling of the distal ends of projecting spicules is not confined to Rhaphidotheca; I have it in a less marked form in a suberite to which I give the name of Radiella schoenus (σχοîυος, a bullrush) … The swollen terminations of the spicules of R. schoenus suggest the possibility of a polyphyletic origin for the Tetractinellida.’ (pp. 162–163). In 1885 Vosmaer described a very similar species as Polymastia capitata. After examination of Sollas's material, Dendy & Ridley (Reference Dendy and Ridley1886) synonymized P. capitata with R. schoenus, the latter becoming the senior synonym, but retained this species in Polymastia. Despite the act by Dendy and Ridley, Topsent (Reference Topsent1898) erected a new genus, Sphaerotylus, for P. capitata but not for R. schoenus. However, later (Topsent, Reference Topsent1913) he acknowledged the seniority of R. schoenus. Meanwhile, Kirkpatrick (Reference Kirkpatrick1908) considered R. schoenus as a nomen nudum. Since then both names, S. schoenus and S. capitatus (occasionally allocated to Polymastia), have been used in different papers (e.g. Topsent, Reference Topsent1928; Koltun, Reference Koltun1966; Boury-Esnault, Reference Boury-Esnault, Hooper and van Soest2002; Plotkin, Reference Plotkin, Pansini, Pronzato, Bavestrello and Manconi2004). Moreover, sponges found in the southern hemisphere (including the Antarctic) that have similar morphologies, have also been identified under the same names, S. capitatus or S. schoenus (Burton, Reference Burton1929; Koltun, Reference Koltun, Pavlovskii, Andriyashev and Ushakov1964; Boury-Esnault & Van Beveren, Reference Boury-Esnault and van Beveren1982; Uriz, Reference Uriz1988; Barthel et al., Reference Barthel, Tendal and Panzer1990; Sarà et al., Reference Sarà, Balduzzi, Barbieri, Bavestrello and Burlando1992). Formally R. schoenus cannot be regarded as nomen nudum since Sollas mentioned at least one feature of it, although his description is extremely poor. Nevertheless, for stability reasons we follow Boury-Esnault (Reference Boury-Esnault, Hooper and van Soest2002) and accept the name S. capitatus as valid since it has been used more frequently than S. schoenus in the last decades. We also agree with her that the records of S. capitatus/S. schoenus from the southern hemisphere should be regarded as another species. These records are gathered under the species name S. vanhoeffeni Hentschel, Reference Hentschel and von Drygalski1914 below.
Sphaerotylus exospinosus Lévi, Reference Lévi and Crosnier1993
(Figure 15)

Fig. 15. Sphaerotylus exospinosus, spicules on the type slide MNHN D-CL 3583: (A) principal subtylostyle; (B) intermediary tylostyles; (C) small tylostyles; (D) fully developed exotyles; (E) not fully developed exotyle, general view; (F) proximal tip of the exotyle depicted in E, detailed view; (G) distal knob of the exotyle depicted in E, detailed view. Scale bars: A–G, 0.1 mm.
Original description: Sphaerotylus exospinosus Lévi, Reference Lévi and Crosnier1993, p. 25, figure 6c.
TYPE MATERIAL
Holotype: MNHN D-CL 3583 (specimen in alcohol), New Caledonia, SW Pacific, 22°53.05′S 167°17.08′E, 570–610 m; BIOCAL campaign on RV ‘Jean Charcot’ in 1985, station DW 46. Lévi based his description on a small sponge fragment which was completely used for making preparations. We have examined these microscopic slides.
DESCRIPTION
External morphology
(according to Lévi, Reference Lévi and Crosnier1993)
Holotype was a piece of a cushion-shaped sponge. Its surface was greyish-pale yellow, hispid because of protruding knobs of exotyles, without papillae.
Skeleton
(according to Lévi, Reference Lévi and Crosnier1993)
Main choanosomal skeleton was composed of longitudinal tracts of principal spicules which extended to the cortex. The cortex comprised a palisade of small spicules and an inner layer of transversal bundles of intermediary spicules. Exotyles rose from the choanosome, crossed the cortex and formed a superficial hispidation actually composing the major portion of the sponge skeleton.
Spicules
(our data, N = 3 for not fully developed exotyles, N = 10 for other categories)
-
• Principal spicules – straight, slightly fusiform subtylostyles (Figure 15A). Length 418–484–622 µm, diameter of tyle 6.5–7.8–9.1 µm, proximal diameter of shaft 3.9–5.1–5.2 µm, maximum diameter of shaft 10.4–12.7–15.6 µm.
-
• Intermediary spicules – gently curved or straight, fusiform tylostyles (Figure 15B). Length 244–307–449 µm, diameter of tyle 7.8–9.6–13.0 µm, proximal diameter of shaft 5.2–6.0–7.8 µm, maximum diameter of shaft 11.7–13.1–15.6 µm.
-
• Small spicules – gently curved, fusiform tylostyles (Figure 15C). Length 93–103–117 µm, diameter of tyle 5.2–5.8–6.5 µm, proximal diameter of shaft 2.6–2.9–3.9 µm, maximum diameter of shaft 3.9–4.7–5.2 µm.
-
• Fully developed exotyles (Figure 15D) 745–926–1041 µm long, with well-developed proximal tyles (13.0–15.3–18.2 µm in diameter, Figure 31F), gradually expanding from 7.8–10.8–13.0 µm (shaft diameter near tyle) to 39.0–46.5–51.9 µm (shaft diameter near distal knob). Distal knobs (62.3–72.2–80.5 µm in diameter) cauliflower-shaped, i.e. the widened distal tip is ornamented by a dense crown of branching protuberances. Shaft under the main ornamentation often with small tubercules.
-
• Not fully developed exotyles of the same shape as the fully developed ones, but smaller. Length 500–571–633 µm, diameter of tyle 10.4–11.7–13.0 µm, proximal diameter of shaft ~ 8 µm, distal diameter of shaft 20.8–27.7–31.2 µm, diameter of distal knob 33.8–44.1–51.9 µm (Figure 15E–G).
OCCURRENCE

Fig. 16. Distribution of Polymastiidae with ornamented exotyles in the Pacific: square, Sphaerotylus exospinosus; circle, Sphaerotylus exotylotus; cross, Sphaerotylus raphidophora; star, Sphaerotylus sceptrum; trefoil, Sphaerotylus verenae; heart, Tylexocladus hispidus.
Known only from the type locality off New Caledonia, SW Pacific.
REMARKS
Lévi (Reference Lévi and Crosnier1993) established Sphaerotylus exospinosus based on the uniqueness of the cauliflower-shaped ornamentations of its exotyles. However, except for this feature no data on its similarities to and distinctions from other Sphaerotylus spp. can be obtained because of the lack of tissue material.
Sphaerotylus exotylotus Koltun, Reference Koltun and Bogorov1970
(Figures 17 & 18)

Fig. 17. Sphaerotylus exotylotus: (A) lectotype ZIN RAS 10615, habitus; (B) and (C) paralectotypes ZIN RAS 10615, habitus; (D) surface of the lectotype, detailed view; (E) longitudinal section through the body of the lectotype. Scale bars: A–C, 10 mm; D, 0.2 mm; E, 1 mm.

Fig. 18. Sphaerotylus exotylotus, spicules: (A) long principal subtylostyle, general view; (B) proximal tip of the subtylostyle depicted in A, detailed view; (C) distal tip of the subtylostyle depicted in A, detailed view; (D) short principal subtylostyle, general view; E, proximal tip of the subtylostyle depicted in D, detailed view; (F) distal tip of the subtylostyle depicted in D, detailed view; (G) intermediary tylostyle; (H) small tylostyles; (I) exotyle. Scale bars: A, 0.2 mm; B and C, 0.01 mm; D, 0.1 mm; E and F, 0.01 mm, G, 0.1 mm; H, 0.02 mm; I, 0.1 mm.
Original description: Sphaerotylus exotylotus Koltun, Reference Koltun and Bogorov1970, p. 175, pl. VII figures 1 & 2, text-figure 7.
SYNONYMS AND CITATIONS
Sphaerotylus exotylotus (Plotkin, Reference Plotkin2002, p. 106, figure 3.)
TYPE MATERIAL
Lectotype (designated herein, see Figure 17A): ZIN RAS 10615 (specimen in alcohol), slide 16160), Simushir Island, Kurile Islands, NE Pacific, 46°38′N 152°03′E, 1440–1540 m, RV ‘Vityaz’, cruise 39, station 5594, 12.07.1966.
Paralectotypes (Figure 17B, C): ZIN RAS 10615 (two specimens in alcohol), from the same sample as the lectotype.
DESCRIPTION
External morphology
Small, thick, cushion-shaped sponges detached from substrata (Figure 17A–C). Surface for the most part rough or velvety, knobbly and dark brown in colour (Figure 17D). Each specimen with a single exhalant papilla which in the preserved state is considerably contracted and invaginated into the surface. Area surrounding the papilla free of knobs, wrinkled and light in colour. Lectotype 2.4 × 1.5 × 0.6 cm in size, with the smooth area around its papilla occupying ~ 1/3 of the surface (Figure 17A). One of the paralectotypes 1.5 × 0.8 × 0.3 cm in size, with the smooth area around its papilla slightly reduced (Figure 17B). The other paralectotype 0.8 × 0.6 × 0.2 cm in size, with the smooth area hardly visible with the naked eye (Figure 17C).
Skeleton
Main choanosomal skeleton composed of radial tracts of principal spicules which enter the cortex (Figure 17E). Auxiliary choanosomal skeleton comprises singly scattered small and intermediary spicules and occasionally exotyles. Dense superficial cortical palisade made of exotyles, between which small spicules are embedded. Internal cortical layer of criss-cross intermediary spicules confused, loose and disrupted by the exotyles.
Spicules
(measurements based on three specimens, N = 30)
-
• Principal spicules – usually straight, slightly fusiform subtylostyles (Figure 18A–F). Length 700–1183–1700 µm, diameter of shaft 15.0–19.2–25.0 µm.
-
• Intermediary spicules – gently curved, slightly fusiform tylostyles (Figure 18G). Length 200–326–500 µm, diameter of shaft 8.2–11.3–14.0 µm.
-
• Small spicules – straight or gently curved, slender tylostyles (Figure 18H). Length 100–138–180 µm, diameter of shaft 5.1–6.8–8.0 µm.
-
• Exotyles straight, clavate, 500–668–850 µm long (Figure 18I). Proximal tyles usually well-developed, occasionally weakly developed, 18.5–23.6–30.0 µm in diameter. Distal knobs well-developed, regular, bulb- or pear-shaped, with rough, spined or granulated surface, 80.2–97.8–110.0 µm in diameter.
REMARKS
Sphaerotylus exotylotus resembles S. vanhoeffeni, especially in the substitution of the palisade of exotyles for the ordinary palisade of tylostyles and the inner layer of criss-cross spicules in the cortex, but differs by the peculiar clavate shape of the exotyles.
Sphaerotylus isidis (Thiele, Reference Thiele1905) comb. nov.
(Figures 19 & 20)

Fig. 19. Sphaerotylus isidis: (A) lectotype ZMB 3271, habitus; (B)–(E), paralectotypes, ZMB 3271, habitus; (F) longitudinal section through the body of the lectotype, general view; (G) the same section, detailed view of cortex; (H) transversal section through a papilla of the paralectotype depicted in B; (I) longitudinal section through another papilla of the same paralectotype. Scale bars: A–E, 10 mm; F, 1 mm; G and H, 0.5 mm; I, 1 mm.

Fig. 20. Sphaerotylus isidis, spicules: (A) principal styles; (B) intermediary subtylostyle; (C) small tylostyle; (D) exotyle with rounded distal tip, general view; (E) proximal tip of the exotyle depicted in D, detailed view; (F) distal tip of the exotyle depicted in D, detailed view; (G) exotyle with slightly irregular, spherical distal knob, general view; (H) proximal tip of the exotyle depicted in G, detailed view; (I) distal knob of the exotyle depicted in G, detailed view; (J) exotyle with regularly spherical distal knob, general view; (K) proximal tip of the exotyle depicted in J, detailed view; (L) distal knob of the exotyle depicted in J, detailed view. Scale bars: A and B, 0.1 mm; C, 0.01 mm; D, 0.1 mm; E and F, 0.01 mm; G, 0.1 mm; H and I, 0.01 mm; J, 0.1 mm, K and L, 0.01 mm.
Original description: Polymastia isidis (Thiele, Reference Thiele1905, p. 414, figures 25 and 38a–e).
SYNONYMS AND CITATIONS
Nec Polymastia isidis (Burton, Reference Burton1932, p. 337; Koltun, Reference Koltun, Pavlovskii, Andriyashev and Ushakov1964, p. 26; Desqueyroux, Reference Desqueyroux1975, p. 57; Boury-Esnault & Van Beveren, Reference Boury-Esnault and van Beveren1982, p. 35, pl. 4 figure 15; Uriz, Reference Uriz1988, p. 44, figure 20a–c).
Nec Polymastia isidis var. simplex Hentschel, Reference Hentschel and von Drygalski1914, p. 47, pl. V figure 3.
TYPE MATERIAL
Lectotype (designated herein, see Figure 19A): ZMB 3271 (specimens in alcohol), Almirantazgo Sound (Admiralty Sound), Tierra del Fuego, Chilean Coast, SE Pacific, 54°19.0′S 69°30.0′W, 19 m, coll. Plate.
Paralectotypes (Figure 19B–E): ZMB 3271 (four specimens in alcohol), from the same sample as the holotype.
DESCRIPTION
External morphology
Encrusting sponges with prominent cylindrical or slightly conical papillae which lack visible oscula. Surface mostly rough, dirty greyish in colour, but partly smooth and pale. Lectotype 3.6 × 2.3 cm in size, with 17–18 papillae, attached to a bivalve shell (Figure 19A). Paralectotypes with less rough surface, attached to pebbles and/or to shell fragments. The largest paralectotype 4.3 × 2.9 cm in size, with ~ 26 papillae (Figure 19B). Other paralectotypes damaged (Figure 19C–E).
Skeleton
Main choanosomal skeleton composed of longitudinal or radial tracts of principal spicules entering the cortex and partly protruding above it (Figure 19F). Auxiliary choanosomal skeleton formed by scattered intermediary and small spicules, the latter usually arranged in dense bundles of up to 10 spicules each. Cortex comprises a palisade (~ 110 µm thick) of small spicules and an inner layer (70–80 µm thick) of tangentially arranged intermediary spicules, separated by a distinct zone (~ 180 µm thick) with few spicules (Figure 19G). Exotyles sparsely scattered over the cortex rising above the palisade. Both cortical layers extend to the papillae walls (Figure 19H, I). Bulkheads between the canals reinforced by the intermediary spicules (Figure 19H).
Spicules
(measurements based on lectotype and two paralectotypes, N = 15 for exotyles, N = 30 for other categories)
-
• Principal spicules – straight, slender subtylostyles with displaced tyles, often polytylote and with rounded distal tips (Figure 20A). Length 679–751–818 µm, diameter of tyle 8.8–13.5–17.9 µm, proximal diameter of shaft 7.5–8.6–10.2 µm, maximum diameter of shaft 12.9–15.2–17.9 µm.
-
• Intermediary spicules – straight subtylostyles to tylostyles (Figure 20B). Length 400–418–448 µm, diameter of tyle 8.2–9.0–9.9 µm, proximal diameter of shaft 5.4–7.5–9.5 µm, maximum diameter of shaft 10.1–11.2–12.3 µm.
-
• Small spicules – straight or gently curved, slender tylostyles to subtylostyles (Figure 20C). Length 106–160–210 µm, diameter of tyle 4.7–6.8–8.1 µm, proximal diameter of shaft 3.2–4.9–7.2 µm, maximum diameter of shaft 4.0–6.3–8.2 µm.
-
• Exotyles usually gently curved, slightly fusiform (Figure 20D, G, J). Length 682–863–1085 µm, maximum diameter of shaft 12.9–15.6–18.8 µm. Proximal tyles weakly developed, occasionally displaced or absent (Figure 20E, H, K). Some exotyles with extra tyles along the shafts (Figure 20G). Distal knobs (diameter 11.7–13.8–15.5 µm) mostly of regularly spherical shape, more rarely slightly irregular, with granulated surface (Figure 20I, L). Occasionally the knob is absent, and an exotyle terminates with a gradually expanded blunt distal tip (Figure 20F).
OCCURRENCE
(Figure 3)
Known only from the type locality off the Chilean coast, SE Pacific. Records from other regions need verification.
REMARKS
We transfer isidis from Polymastia to Sphaerotylus since the type specimens possess exotyles with spherical distal knobs, which is the main diagnostic feature of the type species of Sphaerotylus. Meanwhile, neither the author of S. isidis (Thiele, Reference Thiele1905), nor the early investigators of the type material (Desqueyroux-Faúndez & Van Soest, Reference Desqueyroux-Faúndez and van Soest1997) noted the exotyles. Evidently they made preparations only from the edge parts of the sponges where the exotyles were damaged. Comparing S. isidis with their new species Polymastia villosa Desqueyroux-Faúndez & van Soest (Reference Desqueyroux-Faúndez and van Soest1997) wrote: ‘We have also examined the holotype (here designated) ZMB 3267, of Polymastia isidis Thiele, Reference Thiele1905, from Chile, which is distinct from our new species in the size of the largest tylostyles, which reach only 850 × 15 µm. That species was also reported from Kerguelen (Boury-Esnault & Van Beveren, Reference Boury-Esnault and van Beveren1982) with larger tylostyles (up to 1600 µm) and with several papillae; this may turn out to be a separate species’ (p. 421). This refers to the designation of the lectotype, but it is unclear which of the syntypes they had examined because there was no picture or text passage indicating which specimen the measurements were based on. Following the original description the species name Polymastia isidis appeared repeatedly in the records of sponges from various areas in the southern hemisphere other than the type locality near the Chilean coast, – Wilhelm II coast of the Antarctica (Hentschel, Reference Hentschel and von Drygalski1914), Palmer Archipelago and Falkland Islands (Burton, Reference Burton1932), South Shetland Islands (Desqueyroux, Reference Desqueyroux1975), Kerguelen (Boury-Esnault & Van Beveren, Reference Boury-Esnault and van Beveren1982), Namibian coast (Uriz, Reference Uriz1988) and eastern Weddell Sea (Barthel et al., Reference Barthel, Tendal and Panzer1990). However, none of these authors mentioned the exotyles in their sponges and it therefore remains uncertain whether they belong to S. isidis or not. For the moment we can only confirm the absence of exotyles in one of the syntypes of Polymastia isidis var. simplex Hentschel, Reference Hentschel and von Drygalski1914 (ZMB 4829) which we have studied. Other records need verification.
Sphaerotylus raphidophora Austin, Ott, Reiswig, Romagosa & McDaniel, Reference Austin, Ott, Reiswig, Romagosa and McDaniel2014
Original description: Sphaerotylus raphidophora Austin, Ott, Reiswig, Romagosa & McDaniel, Reference Austin, Ott, Reiswig, Romagosa and McDaniel2014, p. 36, figures 12 & 13.
TYPE MATERIAL
(not studied)
Holotype: USNM 1231336, Giacomini Seamount, Gulf of Alaska, NE Pacific, 56°25.43′N 146°22.28′W), 862 m, NOAA 2004 Exploring Alaska's Seamounts Expedition, Alvin Dive 4040, 16.08.2004.
DESCRIPTION
(according to Austin et al., Reference Austin, Ott, Reiswig, Romagosa and McDaniel2014)
External morphology
Irregular button-shaped sponge ~ 1.6–1.7 cm in diameter and 0.69 cm thick. Surface yellow-brown in alcohol. No papillae observed.
Skeleton
Main choanosomal skeleton composed of longitudal tracts of principal spicules. Auxiliary choanosomal skeleton comprises singly scattered intermediary spicules and occasional trichodragmata of raphides. Cortex formed by a palisade of small spicules reinforced by exotyles.
Spicules
(see Austin et al. (Reference Austin, Ott, Reiswig, Romagosa and McDaniel2014) for number of spicules measured)
-
• Principal spicules – straight, fusiform subtylostyles or strongyloxeas, occasionally with rounded distal extremities. Length 711–1107–1615 µm, diameter 10.3–20.4–25.4 µm.
-
• Intermediary spicules – gently curved, fusiform tylostyles. Length 228–418–613 µm, diameter 10.5–13.4–17.8 µm.
-
• Small spicules – gently or considerably curved, fusiform tylostyles to styles. Length 104–172–271 µm, diameter 2.0–3.6–6.6 µm.
-
• Raphides often with furcate extremities and numerous procumbent processes along the shaft. Length 60.8–72.4–80.
-
• Exotyles straight, with rounded smooth proximal extremities and rounded granulated distal extremities, occasionally with weakly developed distal knobs. Length 568–890–1374 µm, diameter 26.0–38.9–49.9 µm.
REMARKS
Sphaerotylus raphidophora is distinguished from all other Sphaerotylus spp. by the presence of raphides in trichodragmata that is in fact the main diagnostic feature of Spinularia Gray, Reference Gray1867. Sphaerotylus raphidophora and the type species of Spinularia, S. spinularia (Bowerbank, Reference Bowerbank1866), also possess the similar architechure of cortex formed by a single layer, a palisade of small spicules. At the same time Spinularia spp. lack exotyles and possess a marginal spicule fringe (Plotkin et al., Reference Plotkin, Gerasimova and Rapp2012) that is absent in S. raphidophora. External morphology of S. raphidophora and its exotyles with rounded tuberculated distal extremities resemble those of S. capitatus and S. isidis, although the distal swellings on the exotyles of the latter two species are more prominent. For a full description of S. raphidophora see Austin et al. (Reference Austin, Ott, Reiswig, Romagosa and McDaniel2014).
Sphaerotylus renoufi sp. nov.
(Figures 21 & 22)

Fig. 21. Sphaerotylus renoufi: (A) holotype BELUM MC5015, habitus; (B) paratype BELUM MC5010, habitus; (C) specimen BELUM MC5068 in situ on Thumb Rock, Mullaghmore, Co Sligo, NW Ireland (courtesy of B. Picton, Ulster Museum, Belfast); (D) longitudinal section through the body of paratype BELUM MC5013, general view; (E) the same section, detail of cortex echinated by an exotyle; (F) longitudinal section through the body of holotype BELUM MC5015, detailed view of cortex; (G) longitudinal section through a papilla of the holotype stained with toluidine. Scale bars: A and B, 10 mm;D, 1 mm; E, 0.5 mm; F, 0.2 mm; G, 1 mm.

Fig. 22. Sphaerotylus renoufi, spicules: (A) and (B) subtylostyles, general view; (C) proximal tip of the subtylostyle depicted in B, detailed view; (D) distal tip of the subtylostyle depicted in B, detailed view; (E) intermediary subtylostyles; (F) small tylostyles; (G) exotyle, general view; (H) proximal tip of the exotyle depicted in G, detailed view; (I) fungiform distal ornamentation of the exotyle depicted in G, detailed view; (J) proximal tip and (K) grapnel-like distal ornamentation of another exotyle, detailed view; (L) proximal tip and (M) rounded distal ornamentation of one more exotyle, detailed view. Scale bars: A and B, 0.1 mm; C and D, 0.01 mm; E, 0.1 mm; F, 0.05 mm; G, 0.5 mm; H–M, 0.01 mm.
TYPE MATERIAL
Holotype (Figure 21A): BELUM MC5015 (in alcohol), Glannafeen Cliff, Lough Hyne, Co Cork, SW Ireland, 51°30.03′N 09°18.12′W, 10 m, 25.05.2009, coll. B.E. Picton.
Paratype: BELUM MC5010 (one specimen in alcohol, Figure 21B), from the same locality as the holotype.
Paratype: BELUM MC5013 (one specimen in alcohol), from the same locality as the holotype.
COMPARATIVE MATERIAL EXAMINED
South-West Ireland (eight specimens):
BELUM MC7695, Mc7696 and MC7697 (three specimens), Co Cork, Lough Hyne, Glannafeen Cliff, 51°30.03′N 09°18.12′W, 6–10 m, 02.–03.08.1993, coll. C.C. Morrow & B.E. Picton. BELUM MC3708 and MC3711 (two specimens), Co Cork, Lough Hyne, Glannafeen Cliff, 51°30.03′N 09°18.12′W, 10 m, 09.04.2007, coll. B.E. Picton. BELUM MC7698 (one specimen), Co Cork, Bantry Bay, S of Black Ball Head, 51°35.31′N 10°02.22′W, 35 m, 05.06.1993, coll. B.E. Picton. BELUM MC7699 (one specimen), Co Kerry, Kenmare River, Kilmakillogue Harbour, 51°46.64′N 09°49.77′W, depth 20 m BCD; coll. B.E. Picton, 12.08.1995. BELUM MC7700 (one specimen), Co Kerry, Kenmare River, NE of Inishkeragh, 51°47.94′N 09°53.29′W, 21 m, 13.08.1995, coll. E.M. Sides.
West Ireland (four specimens):
BELUM MC7701 (two specimens), Co Galway, Mannin Bay, Carrigeenbeg, 53°26.75′N 10°12.75′W, 40 m, coll. C.C. Morrow, 16.06.1995. BELUM Mc7702 (one specimen), Co Galway, Clifden Bay, SSW of Carrickana Rocks, 53°28.98′N 10°09.93′W, 38 m, coll. B.E. Picton, 11.06.1995. BELUM Mc7703 (one specimen), Co Galway, Friar Island, N of Malthooa, 53°33.23′N 10°13.57′W, 34 m, coll. B.E. Picton, 22.06.1995.
North-West Ireland (10 specimens):
BELUM Mc7705 (one specimen), Co Mayo, Inishkea Island, 54°04.36′N 10°11.98′W, 43 m, coll. B.E. Picton, 08.08.1994. BELUM Mc7706 (one specimen), Co Sligo, Mullaghmore, Thumb Rock, 54°28.31′N 08°26.71′W, 22 m, 16.05.1994, coll. C.C. Morrow. BELUM Mc7707 (one specimen), Co Donegal, St. John's Point, Black Rock, 54°34.69′N 08°25.64′W, 19 m, 22.05.1994, coll. C.C. Morrow. BELUM Mc7708 (one specimen), Co Donegal, SE Deegagh Point, 55°09.23′N 07°41.55′W, 12 m, 13.07.1993, coll. C.C. Morrow. BELUM Mc5056, Mc5061, Mc5068, Mc5073, Mc5076 and Mc5080 (six specimens), Co Sligo, Mullaghmore, Thumb Rock, 54°28.31′N 08°26.71′W, 22 m, 8.–10.07.2009, coll. B.E. Picton & C.C. Morrow.
North-East Ireland (one specimen):
BELUM Mc3761, Co Antrim, Rathlin Island, Duncan's Bay, 55°18.70′N 06°15.09′W, 34 m 22.06.2007, coll. B.E. Picton.
Irish Sea, Welsh Coast (six specimens):
BELUM Mc5428, Mc5435, Mc5440 and Mc5441 (four specimens), Pembrokeshire coast, Huw's Reef, 51°57.84′N 05°07.54′W, 17.4 m, coll. B.E. Picton, 04.08.2009. BELUM Mc5757 and Mc5760 (two specimens), Pembrokeshire coast, Skomer, Thorn Rock, 51°43.80′N 5°15.95′W, 18.8 m, 06.08.2009, coll. B.E. Picton.
ETYMOLOGY
Named after Professor Louis Renouf of University College, Cork, the first biologist to note the unique character of Lough Hyne, Co Cork and to begin marine research there in 1923.
DESCRIPTION
External morphology
Cushion-shaped sponges with a convex upper surface (Figure 21A–C). Surface shaggy, dark in colour because of the covering silt, with bright yellow papillae (in life, Figure 21C). Papillae with oscula on the summits. Holotype 1.6 × 1.5 × 0.4 cm in size, with four papillae which are 3–6 mm long and ~ 2 mm in diameter (Figure 21A). Other specimens up to 12 cm2, with one to five papillae per cm2 of the surface. Papillae 1–11 mm long and 1.5–3.5 mm in diameter.
Skeleton
Main choanosomal skeleton composed of radial or longitudinal tracts (~ 110 µm thick) of principal spicules which cross the cortex and make up a surface hispidation that is up to 2200 µm thick (Figure 21D). Auxiliary choanosomal skeleton comprises singly scattered small and intermediary spicules. Cortex up to 300 µm thick composed of an outer layer of small spicules arranged in bouquets and a slightly thinner, loose inner layer of tangentially arranged intermediary spicules (Figure 21E, F). Exotyles cross the cortex (Figure 21E). Both cortical layers extend to the papillae walls (Figure 21G). Central exhalant canal in papilla surrounded by ascending tracts of principal spicules. Several inhalant canals located in the periphery. Bulkheads between the canals reinforced by a network of intermediary spicules.
Spicules
(measurements based on holotype and two paratypes, N = 19 for exotyles, N = 70 for other categories)
-
• Principal spicules – usually straight, slightly fusiform, polytylote subtylostyles, often with blunt distal tips (Figure 22A–D). Length 560–796–1030 µm, diameter of shaft 7.5–14.8–16 µm.
-
• Intermediary spicules – straight, slender tylostyles to subtylostyles (Figure 22E). Length 200–415–650 µm, diameter of shaft 5.0–9.7–13.8 µm.
-
• Small spicules (Figure 22F) – straight, slightly fusiform tylostyles. Length 70–132–210 µm, diameter of shaft 2.0–4.1–6.5 µm.
-
• Exotyles gently curved or straight, almost cylindrical, slender (Figure 22G–M). Length 1110–1755–2460 µm, diameter of shaft 5.0–8.0–10.0 µm. Proximal tyles are weakly developed (Figure 22H, J, L) or absent. Distal knobs (7.0–19.4–25.3 µm) fungiform (Figure 22I) or lobate (Figure 22K), occasionally subspherical (Figure 22M), with granulated surface.
OCCURRENCE
NE Atlantic: widely distributed around Ireland (western coast and Irish Sea) and along western Wales (Pembrokeshire coast), 6–42 m.
REMARKS
Sphaerotylus renoufi resembles S. antarcticus and S. borealis in several features – a thick superficial hispidation composed of the ascending tracts of principal spicules, several prominent papillae and a two-layered cortex, but differs from the latter two species by shorter principal spicules and exotyles, as well as by the presence of lobate distal knobs on some exotyles.
Sphaerotylus sceptrum Koltun, Reference Koltun and Bogorov1970
(Figures 17 & 18)
Original description: Sphaerotylus sceptrum Koltun, Reference Koltun and Bogorov1970, p. 177, pl. V figure 4, text-figure 8.
SYNONYMS AND CITATIONS
Sphaerotylus sceptrum (Plotkin, Reference Plotkin2002, p. 106, figure 2).
TYPE MATERIAL
Holotype: ZIN RAS 10614 (specimen in alcohol, slide 16132), Simushir Island, Kurile Islands, NE Pacific, 46°38′N 152°03′E, 1440–1540 m, RV ‘Vityaz’, cruise 39, station 5594, 12.07.1966.
DESCRIPTION
External morphology
Several fragments of a cushion-shaped, crumby sponge detached from substratum. Surface bears tiny papillae with oscula on the summits. Surface areas surrounding the papillae pale and almost smooth. Peripheral surface rough or velvety and brownish in colour. Largest fragment 4 × 3.5 × 1.5 cm in size, with three papillae.
Skeleton
Main choanosomal skeleton composed of radial tracts of principal spicules which ascend and fan in the cortex (Figure 23A). Auxiliary choanosomal skeleton comprises singly scattered small spicules, pairs of exotyles and occasionally intermediary spicules. Cortex around the papillae 1700–2100 µm thick, composed of a superficial layer (150–200 µm thick) of dense bouquets of small spicules reinforced by the branching tracts ascending from the choanosome, a loose inner layer (300–750 µm thick) of criss-cross intermediary spicules and a space with aquiferous cavities in between the spicule layers (Figure 23B, C). The cavities connected with ostia scattered between the superficial spicule bouquets. Bulkheads between the cavities reinforced by the ascending choanosomal tracts of principal spicules and single intermediary spicules. Peripheral cortex is a dense palisade of exotyles, occasionally encrusted with the small spicules and underlain by tufts of the intermediary spicules (Figure 23B, D).

Fig. 23. Sphaerotylus sceptrum, holotype ZIN RAS 10614: (A) longitudinal section through the body, general view; (B) another longitudinal section through the body showing the transitional area between the peripheral and central cortex; (C) the same section, detail of the central cortex showing bouquets of small spicules reinforced by the tracts ascending from choanosome; (D) the same section; detail of the peripheral cortex showing a palisade of exotyles; (E) principal style and exotyle; (F) intermediary subtylostyles; (G) small tylostyle; (H) exotyle. Scale bars: A, 3 mm; B, 0.5 mm; C and D, 0.3 mm; E, 0.1 mm; F, 0.04 mm; G, 0.01 mm; H, 0.02 mm.
Spicules
(N = 10)
-
• Principal spicules – straight, slightly fusiform styles (Figure 23E). Length 600–1254–1400 µm, proximal diameter of shaft 9.2–12.9–15.1 µm, maximum diameter of shaft 15.0–20.3–25.0 µm.
-
• Intermediary spicules – straight, slender or occasionally stout tylostyles to subtylostyles (Figure 23F). Length 200–411–524 µm, proximal diameter of shaft 5.5–9.3–11.2 µm, maximum diameter of shaft 8.0–11.1–13.9 µm.
-
• Small spicules – usually straight, slender tylostyles (Figure 23G). Length 101–128–160 µm, diameter of tyle 4.1–4.4–5.5 µm, proximal diameter of shaft 2.8–3.4–4.5 µm, maximum diameter of shaft 3.7–4.3–5.7 µm.
-
• Exotyles stout, sceptre-shaped (Figure 23E, H). Length 195–219–250 µm. Well-developed proximal tyles, 13.4–16.1–20.1 µm in diameter. Shafts gradually expanding from 10.2–11.4–13.0 µm near the proximal tyles to 28.5–31.9–35.0 µm at the distal extremities. Surface of the distal extremeties turberculated or granulated. No distal knobs.
REMARKS
Sphaerotylus sceptrum is distinguished from its congeners by the remarkably heterogeneous cortex. In the areas around the papillae it is composed of a superficial palisade of small tylostyles and an inner layer of criss-cross spicules, bears ostia and aquiferous cavities and lacks exotyles that is architecture typical of many other polymastiids. However, in the peripheral zones the palisade of exotyles completely substitutes for the layers of tylostyles that resemble the cortex in Sphaerotylus exotylotus and S. vanhoeffeni. The exotyles of S. sceptrum are most similar to those of S. vanhoeffeni, but in the former species they are shorter and expand much more towards the distal extremities which do not bear any knobs and are covered by the remarkably large tubercules.
Sphaerotylus strobilis sp. nov.
(Figures 24 & 25)

Fig. 24. Sphaerotylus strobilis: (A) holotype BMNH 1926.4.14.86.7.517, habitus; (B) paratype BMNH 1926.4.14.86.7.519, habitus; (C) longitudinal section through the body of the holotype, general view; (D) the same section, detail of cortex; (E) longitudinal section through a papilla of the holotype. Scale bars: A and B, 10 mm; C, 1 mm; D, 0.4 mm; E, 1 mm.

Fig. 25. Sphaerotylus strobilis, spicules: (A) principal subtylostyle, general view; (B) proximal tip of the subtylostyle depicted in A, detailed view; (C) distal tip of the subtylostyle depicted in A, detailed view; (D) intermediary subtylostyle, general view; (E) proximal tip of the subtylostyle depicted in D, detailed view; (F) distal tip of the subtylostyle depicted in D, detailed view; (G) small tylostyle; (H) exotyle with a regular distal knob, general view; (I) proximal tip of the exotyle depicted in H, detailed view; (J) distal knob of the exotyle depicted in H, detailed view; (K) exotyle with an irregular distal knob; (L) proximal tip of the exotyle depicted in K, detailed view; (M) distal knob of the exotyle depicted in K, detailed view. Scale bars: A, 0.2 mm; B and C, 0.01 mm; D, 0.1 mm; E and F, 0.01 mm; G, 0.04 mm; H, 0.1 mm; I and J, 0.005 mm; K, 0.1 mm; L and M, 0.005 mm.
TYPE MATERIAL
Holotype: BMNH 1926.4.14.86.7.517 (specimen in alcohol), South Africa, depth unknown, coll. J.D.F. Gilchrist.
Paratype (one specimen in alcohol)): BMNH 1926.4.14.86.7.519, South Africa, depth unknown, coll. J.D.F. Gilchrist.
Both sponges are labelled Proteleia sollasi, presumably by Kirkpatrick.
ETYMOLOGY
The name refers to the shape of the distal knobs of exotyles (Latin strobilus = a strobile, a cone).
DESCRIPTION
External morphology
Both sponges cushion-shaped, attached to bivalves. Holotype (Figure 24A) ~ 3.5 × 3.5 × 1.5 cm in size. Surface minutely hispid, mostly covered by sediment, with sparse clean yellowish areas and nine conical yellowish papillae, 0.4–1.6 cm long, 0.2–0.8 cm wide at base. Paratype (Figure 24B) ~ 3 × 3 × 0.7 cm in size. Surface mostly velvety, free of sediment, yellowish in colour, with a narrow marginal hispidation and eight yellowish papillae. Papillae conical or cylindrical, 1–1.8 cm long, 0.3–0.5 cm wide at base. Considerably contracted oscula visible on the summits of most papillae in both specimens.
Skeleton
Main choanosomal skeleton (Figure 24C) composed of radial or longitudinal tracts (220–450 µm thick) of principal spicules. Tracts radiate and cross the cortex, few of them forming a surface hispidation. Auxiliary choanosomal skeleton mainly of intermediary spicules which are often grouped in dense bundles, each bundle consisting of up to 10 spicules. These bundles are highly abundant in the subcortical area where they cross each other (Figure 24D). Tiny sediment particles and foraminiferans are commonly incorporated in the choanosome. Cortex comprises three layers (Figure 24D) – a superficial palisade (170–290 µm thick) of small spicules, an inner well-defined layer (120–330 µm thick) of densely lying criss-cross intermediary spicules and an intermediate layer (230–400 µm thick) where intermediary spicules are sparsely scattered. Single exotyles scattered among the small spicules in the palisade join the surface hispidation. Skeleton of papillae walls made of the cortical palisade and the inner layer where the criss-cross intermediary spicules distributed more sparsely than in the cortex (Figure 24E).
Spicules
(measurements based on holotype, N = 9 for exotyles, N = 30 for other categories)
-
• Principal spicules – straight, slender, subtylostyles to styles (Figure 25A–C). Length 860–1007–1100 µm, proximal diameter of shaft 7.2–8.4–9.1 µm, maximum diameter of shaft 19.5–21.7–24.3 µm.
-
• Intermediary spicules – styles and subtylostyles resembling principal spicules in shape (Figure 25D–F). Length 490–543–585 µm, proximal diameter of shaft 5.8–6.9–7.3 µm, maximum diameter of shaft 9.8–12.2–14.0 µm.
-
• Small spicules – straight, usually slender tylostyles (Figure 25G). Length 147–170–195 µm, diameter of tyle 4.8–6.4–8.3 µm, proximal diameter of shaft 2.0–3.7–5.1 µm, maximum diameter of shaft 4.9–6.6–8.2 µm.
-
• Exotyles straight or gently curved, fusiform (Figure 25H, K), usually with weakly developed proximal tyles (Figure 25I, L). Length 565–599–632 µm, proximal diameter of shaft 6.2–6.8–7.0 µm, maximum diameter of shaft 14.0–14.5–15.0 µm. Distal tips acerated or blunt, covered by numerous tubercles of different size which usually form regular (Figure 25J), occasionally irregular (Figure 25M), strobile-shaped knobs 6.2–6.9–7.3 µm in diameter.
OCCURRENCE
Known only from the type locality near South Africa.
REMARKS
Holotype and paratype of this new species were labelled as Proteleia sollasi. Presumably the identification was done by Kirkpatrick who studied the ‘Gilchrist collection’ from South Africa (Kirkpatrick, Reference Kirkpatrick1902, Reference Kirkpatrick1903a, Reference Kirkpatrickb), but did not mention these sponges in his papers. In fact Sphaerotylus strobilis lacks at least two main features of P. sollasi, grapnel-like ornamentations on the exotyles and an extra palisade of intermediary spicules in the cortex. At the same time our new species shares the presence of a velvety surface, a three-layered cortex including an intermediate layer of low spicule concentration and a relatively small length of exotyles with S. capitatus and S. isidis. But in contrast to the latter two species in S. strobilis some tracts of principal spicules make up a surface hispidation that rather resembles S. borealis and S. antarcticus, although in the latter two both principal spicules and exotyles are much longer and the hispidation is much more dense and thicker than in our new species. The main distinctive feature of S. strobilis is the strobile-shaped knobs of its exotyles.
Sphaerotylus tjalfei sp. nov.
(Figures 29 & 30)
TYPE MATERIAL
Holotype (specimen in alcohol): ZMUC-DEM-243, West Greenland, 70°47′N 52°21′W, 600 m, RV ‘Tjalfe’, 06.08.1908.
Paratype (one specimen in alcohol): ZMUC-DEM-244 (paratype), from the same sample as the holotype.
Paratype (one specimen in alcohol): ZMUC-DEM-245 (paratype), from the same sample as the holotype.
ETYMOLOGY
‘Tjalfe’ is the name of the Danish hired vessel and the type material was collected during one of her cruises. These specimens were examined by Lundbeck who labelled them ‘Polymastia tjalfi’, but he never described them or mentioned this name in his publications.
DESCRIPTION
External morphology
Dome-shaped sponges with a shaggy surface, dark brown in colour because of the covering silt. Holotype and paratype ZMUC-DEM-244 overgrowing a hard calcareous tube (of a serpulid polychaete or a piece of a hydrocoral skeleton) (Figure 26A). Holotype 2.5 × 2.4 cm in size, bearing a distinct low papilla with an osculum on the summit. Paratype ZMUC-DEM-244 1.9 × 1.6 cm in size, lacking any visible papilla. Paratype ZMUC-DEM-245 1.7 × 1.5 cm in size, detached from substratum and overgrown by an ascidian (Figure 26B). Its single very tiny papilla completely invaginated into the surface hispidation on the body summit.

Fig. 26. Sphaerotylus tjalfei: (A) holotype ZMUC-DEM-243 and paratype ZMUC-DEM-244 growing together, habitus; (B) paratype ZMUC-DEM-245, habitus; (C) exotyle echinating the surface of paratype ZMUC-DEM-245 under stereomicroscope; (D) longitudinal section through the body of paratype ZMUC-DEM-245, general view; (E) the same section, detail of auxiliary choanosomal skeleton; (F) the same section, detail of cortex; (G) the same section, detail of cortex showing stout strongyles. Scale bars: A and B, 10 mm; C, 0.1 mm; D, 1 mm; E and F, 0.5 mm; G, 0.3 mm.
Skeleton
Main choanosomal skeleton composed of radial tracts of principal spicules which cross the cortex and make up a surface hispidation (Figure 26D). Auxiliary choanosomal skeleton comprises singly scattered small spicules (Figure 26E). In cortex a palisade (~ 170 µm thick) of small spicules lies directly on a layer (~ 140 µm thick) of tangentially arranged intermediary spicules (Figure 26F). Short, stout strongyles sparsely scattered along the cortex (Figure 26G). Exotyles cross the cortex joining the surface hispidation (Figure 26C). Distal portions of many protruding spicules are often broken and hence it is impossible to determine whether they are exotyles or usual principal monactines.
Spicules
(measurements based on holotype and both paratypes, N = 5 for exotyles, N = 4 for cortical strongyles, N = 30 for other categories)
-
• Principal spicules – straight or gently curved, fusiform, often polytylote subtylostyles to styles (Figure 27A). Length 854–1273–2013 µm, diameter of tyle (if present) 8.3–13.7–20.8 µm, proximal diameter of shaft 7.5–12.6–20.8 µm, maximum diameter of shaft 19.2–28.2–36.4 µm.

Fig. 27. Sphaerotylus tjalfei, spicules: (A) principal styles; (B) intermediary tylostyle; (C) small tylostyles; (D) exotyle, general view; (E) proximal tip of the exotyle depicted in D, detailed view; (F) distal knob of the exotyle depicted in D, detailed view; (G) and (H) distal knobs of other exotyles, detailed view. Scale bars: A and B, 0.1 mm; C, 0.03 mm; D, 0.1 mm; E–H, 0.01 mm.
-
• Intermediary spicules – usually straight, slender or slightly fusiform tylostyles to subtylostyles (Figure 27B). Length 378–518–797 µm, diameter of tyle 7.8–11.0–19.5 µm, proximal diameter of shaft 5.5–8.4–13.3 µm, maximum diameter of shaft 7.2–14.5–22.6 µm.
-
• Small spicules – straight or occasionally gently curved, stout, fusiform tylostyles (Figure 27C). Length 97–145–226 µm, diameter of tyle 4.1–6.0–8.5 µm, proximal diameter of shaft 3.0–4.5–6.1 µm, maximum diameter of shaft 4.8–7.9–13.7 µm.
-
• Cortical strongyles short, stout, regularly cylindrical or slightly fusiform, occasionally with weakly developed tyles. Length 49–174–314 µm, maximum diameter of shaft 11.9–57.1–90.5 µm.
-
• Exotyles usually gently curved, slender, almost cylindrical (Figure 27D). Length 1080–1710–2856 µm, proximal diameter of shaft 10.5–16.0–19.2 µm, maximum diameter of shaft 17.9–29.4–37.7 µm. Proximal tyles weakly developed or absent (Figure 27E). Distal knobs (18–28.4–37.6 µm in diameter) usually regularly spherical (Figure 27F, G), occasionally with extra swellings on shafts (Figure 27H). Surface of the knobs tuberculated to a greater or lesser extent. Some exotyles lacking distal knobs and only possessing slightly expanded blunt distal tips.
REMARKS
Externally, with its thick surface hispidation and single papilla, Sphaerotylus tjalfei is reminiscent of Polymastia invaginata. But P. invaginata is distinguished by the lack of ornamented exotyles and a cortex composed solely of a palisade of small spicules. The thick surface hispidation along with the two-layered cortex observed in S. tjalfei is also recorded in three other species of Sphaeroylus (S. antarcticus, S. borealis and S. renoufi). However, in contrast to S. tjalfei the latter three species possess several papillae and usually irregular distal knobs on the exotyles. Symmetrically spherical distal knobs on the exotyles of S. tjalfei rather resemble those in the type species of Sphaerotylus, S. capitatus, as well as in S. isidis. Conspicuous stout and short strongyles scattered in the cortex of S. tjalfei are also recorded in P. invaginata by Plotkin & Janussen (Reference Plotkin, Janussen, Martínez Arbizu and Brix2008) and in S. borealis by Swarczewsky (Reference Swarczewsky1906) and Koltun (Reference Koltun1966).
Sphaerotylus vanhoeffeni Hentschel, Reference Hentschel and von Drygalski1914
(Figures 28 & 29)

Fig. 28. Sphaerotylus vanhoeffeni, lectotype ZMB 4837: (A) habitus, general view; (B) habitus, central area of the surface, detailed view; (C) habitus, cut edge, detailed view of exotyle bouquets; (D) distal extremities of the exotyles protruding above the surface, detailed view. Scale bars: A, 2 mm; B and C, 0.5 mm; D, 0.2 mm.

Fig. 29. Sphaerotylus vanhoeffeni, spicules: (A) principal subtylostyles; (B) intermediary tylostyle; (C) small tylostyles; (D) exotyle, general view; (E) proximal tip of the exotyle depicted in D, detailed view; (F) distal knob of the exotyle depicted in D, detailed view; (G) and (H) distal knobs of other exotyles, detailed view. Scale bars: A–D, 0.1 mm; E–H, 0.01 mm.
Original description: Sphaerotylus capitatus var. vanhöffeni Hentschel, Reference Hentschel and von Drygalski1914, p. 50, pl. 5 figure 5.
SYNONYMS AND CITATIONS
Sphaerotylus capitatus (Kirkpatrick, Reference Kirkpatrick1908, p. 18, pl. XII figure 1c, pl. XIII figures 8–13, pl. XIV figures 1–4; Barthel et al., Reference Barthel, Tendal and Panzer1990, p. 122; Sarà et al., Reference Sarà, Balduzzi, Barbieri, Bavestrello and Burlando1992, p. 568).
?Sphaerotylus capitatus (Boury-Esnault & Van Beveren, Reference Boury-Esnault and van Beveren1982, p. 39, figure 9a–c; Uriz, Reference Uriz1988, p. 43).
Sphaerotylus schoenus (Burton, Reference Burton1929, p. 447; Koltun, Reference Koltun, Pavlovskii, Andriyashev and Ushakov1964, p. 28; Barthel et al., Reference Barthel, Tendal and Panzer1990, p. 122; Sarà et al., Reference Sarà, Balduzzi, Barbieri, Bavestrello and Burlando1992, p. 568).
Sphaerotylus schoenus vanhöffeni (Koltun, Reference Koltun1976), p. 168.
TYPE MATERIAL
Lectotype (designated herein, see Figure 28A): ZMB 4837 (specimen in alcohol), Gauss-Station, Davis Sea, Southern Ocean, 66°02′S 89°38′E, 380 m, Deutschen Südpolar-Expedition, 22.12.1902.
Paralectotypes: ZMB 4837 (two specimens in alcohol), from the same locality as the lectotype, 385 m, Deutschen Südpolar-Expedition, 28.01.1903.
COMPARATIVE MATERIAL EXAMINED
BMNH 1908.2.5.111–112 (one specimen in alcohol and its buds mounted on slide, identified as Sphaerotylus capitatus by Kirkpatrick, Reference Kirkpatrick1908), Flagon Point, Winter Quarters Bay, McMurdo Sound, Ross Sea, Southern Ocean, 77°50′42.77″S 166°39′1.41″E, 18–36 m (10–20 fathoms), British National Antarctic Expedition on RV ‘Discovery’, 21.01.1903.
DESCRIPTION
External morphology
All type specimens cushion-shaped. Lectotype 1.3 × 1.2 × 0.3 cm in size, attached to a concretion fouled by a dead bryozoan (Figure 28A). Surface whitish to dirty greyish in colour, with prominent distal tips of exotyles (Figure 28B–D). A considerable invagination in the central area obviously indicates the position of a papilla in the living sponge (Figure 28B). Paralectotypes considerably damaged in their central areas; one specimen free, 0.3 cm in diameter, the other 0.5 cm in diameter, attached to a pebble. BMNH specimen thickly encrusting, with a roughly velvety, knobbly surface bearing several threads with buds and seven papillae partially invaginated into the surface hispidation.
Skeleton
Main choanosomal skeleton composed of radial tracts of principal spicules which enter the cortex. Auxiliary choanosomal skeleton consists of singly scattered small and intermediary spicules and occasional exotyles. Dense cortex made of exotyle bouquets with sparsely embedded small and intermediary spicules (Figure 28C).
Spicules
(measurements based on three specimens, N = 10)
-
• Principal spicules – straight, slightly fusiform or slender, occasionally polytylote subtylostyles (Figure 29A). Length 936–1179–1489 µm, diameter of tyle 11.1–13.7–17.5 µm, proximal diameter of shaft 7.2–9.6–13.5 µm, maximum diameter of shaft 15.8–19.7–23.6 µm.
-
• Intermediary spicules – almost straight, usually fusiform, stout tylostyles (Figure 29B). Length 280–391–601 µm, diameter of tyle 8.7–9.2–10.1 µm, proximal diameter of shaft 5.3–6.1–7.5 µm, maximum diameter of shaft 12.5–13.4–15.1 µm.
-
• Small spicules – straight or occasionally curved, slender or slightly fusiform tylostyles (Figure 29C). Length 97–123–152 µm, diameter of tyle 4.9–5.7–7.0 µm, proximal diameter of shaft 3.5–4.3–5.3 µm, maximum diameter of shaft 4.5–6.2–8.3 µm.
-
• Exotyles straight, club-shaped, 671–911–1075 µm long (Figure 29D). Proximal tyles weakly developed (Figure 29E). Shafts gradually expanding from 8.5–11.6–17.2 µm at the proximal ends to 21.7–50.5–62.0 µm at the distal extremities (Figure 29D). Distal knobs not much wider than the shaft but well-recognizable due to their strongly tuberculated surface (Figure 29F–H).
OCCURRENCE
(Figure 3)
Southern Ocean: continental sectors 2, 3 (Davis Sea), 5 (Ross Sea), 9 (Weddell Sea) (sectors numbered according to Sarà et al., Reference Sarà, Balduzzi, Barbieri, Bavestrello and Burlando1992), 18–400 m. Indian Ocean: Kerguelen, 234–245 m (data from Boury-Esnault & Van Beveren, Reference Boury-Esnault and van Beveren1982, dubious taxonomic status). SE Atlantic: Namibian Coast, 232–403 m (data from Uriz, Reference Uriz1988, dubious taxonomic status).
REMARKS
Sphaerotylus vanhoeffeni is morphologically very similar to S. capitatus from the northern hemisphere and hence many authors regarded these two as a single species with a bipolar distribution (Kirkpatrick, Reference Kirkpatrick1908; Burton, Reference Burton1929; Koltun, Reference Koltun, Pavlovskii, Andriyashev and Ushakov1964, Reference Koltun1976; Boury-Esnault & Van Beveren, Reference Boury-Esnault and van Beveren1982; Uriz, Reference Uriz1988; Sarà et al., Reference Sarà, Balduzzi, Barbieri, Bavestrello and Burlando1992). In fact the Antarctic sponges differ from the typical S. capitatus by the substitution of the exotyle bouquets for the ordinary cortical palisade and layer of criss-cross tylostyles and the weaker prominence of the distal knobs on the exotyles. Besides that S. vanhoeffeni produces buds that have never been recorded in S. capitatus. However, we have not examined the Kerguelen and South African specimens described by Boury-Esnault & van Beveren (Reference Boury-Esnault and van Beveren1982) and Uriz (Reference Uriz1988), and thus we allocate them to S. vanhoeffeni with some doubt.
Sphaerotylus verenae Austin, Ott, Reiswig, Romagosa & McDaniel, Reference Austin, Ott, Reiswig, Romagosa and McDaniel2014
Original description: Sphaerotylus verenae Austin, Ott, Reiswig, Romagosa & McDaniel, Reference Austin, Ott, Reiswig, Romagosa and McDaniel2014, p. 39, figure 14.
TYPE MATERIAL
(not studied)
Holotype: RBCM (Royal British Columbia Museum in Victoria, British Columbia) 009-00053-001, Endeavour Ridge, off British Columbia/Washington, NE Pacific, 47°48.5′N 129°07.5′W, 2220 m, Alvin Dive A1443, 29.08.1984, coll. V. Tunnicliffe.
Paratype (one specimen): CMNI (Canadian Museum of Nature in Ottawa, Ontario) 2009-0027, Endeavour Ridge, off British Columbia/Washington, NE Pacific, 47°57.6′N 129°06.4′W, 2150 m, KML (Khoyatan Marine Laboratory in North Saanich, British Columbia) 1033, Alvin Dive A1439, 25.08.1984, coll. V. Tunnicliffe.
COMPARATIVE MATERIAL
(not studied)
Two specimens, Endeavour Ridge, off British Columbia/Washington, NE Pacific, 47°57.6′N 129°06.4′W, 2150 m, KML 1033, Alvin Dive A1439, 25.081984, coll. V. Tunnicliffe. One specimen, Rift Valley Floor, 47°55′N 129°06′W, off British Columbia/Washington, NE Pacific, 2196 m, KML 1034, Alvin Dive A1436, 22.08.1984, coll. V. Tunnicliffe.
DESCRIPTION
(according to Austin et al., Reference Austin, Ott, Reiswig, Romagosa and McDaniel2014)
External morphology
Sponges flattened, button-shaped or hemispherical, with single short exhalant papillae, 0.9–2.0 cm in diameter. Surface with smooth central area, white in life and becoming yellowish after preservation, and with a slightly hispid dark brown peripheral band.
Skeleton
Main choanosomal skeleton composed of longitudinal tracts of principal spicules extending to the cortex. Auxiliary choanosomal skeleton unknown. A superficial palisade of small spicules spreads over the entire cortex. In peripheral area it is underlaid by a tangential layer of small and intermediary spicules and reinforced by exotyles.
Spicules
(see Austin et al. (Reference Austin, Ott, Reiswig, Romagosa and McDaniel2014) for number of spicules measured)
-
• Principal spicules – straight, slightly fusiform subtylostyles, often with oval tyles. Length 870–1023–1500 µm, diameter 9.6–17.5–21.1 µm.
-
• Intermediary spicules – straight, slightly fusiform tylostyles. Length 280–531–670 µm, diameter 7.5–11.6–17.5 µm.
-
• Small spicules – gently curved, slightly fusiform tylostyles, occasionally with oval tyles. Length 96–114–142 µm, diameter 2.4–4.0–5.5 µm.
-
• Exotyles club-shaped gradually expanding towards the distal ends, with stronger or weaker developed proximal tyles and rounded smooth distal extremities, occasionally with weakly developed distal swellings. Length 1008–1275–1459 µm, medial diameter 19–48–67 µm.
REMARKS
Sphaerotylus verenae strongly resembles S. exotylotus in external morphology and the club-like shape of the exotyles. Taking into account that both species inhabit deep-sea geothermally active mountainous bottoms of the North Pacific (North-east and North-west region respectively) we can assume their close affinities. The differences between S. verenae and S. exotylotus concern the size and the fine details of exotyles along with the architecture of cortex. Exotyles in the latter species possess well-developed minutely tuberulated distal bulbs and are almost two times shorter than the exotyles in S. verenae which have smooth distal extremities often lacking bulbs. Ordinary polymastiid cortical palisade of small tylostyles found in S. verenae is substituted by a palisade of exotyles in S. exotylotus. For a full description of S. verenae see Austin et al. (Reference Austin, Ott, Reiswig, Romagosa and McDaniel2014).
Genus Trachyteleia Topsent, Reference Topsent1928
TYPE SPECIES
Trachyteleia stephensi Topsent, Reference Topsent1928 (by monotypy).
DIAGNOSIS
Thickly encrusting sponges. Papillae unknown. Main choanosomal skeleton made of radial tracts of principal tylostyles. Auxiliary choanosomal skeleton comprises free-scattered intermediary tylostyles. Cortex composed of a palisade of small tylostyles and an inner layer of criss-cross intermediary tylostyles, and reinforced by exotyles which differ from principal tylostyles only by larger size and finely spined distal extremities.
Trachyteleia stephensi Topsent, Reference Topsent1928
(Figure 30)

Fig. 30. Trachyteleia stephensi, holotype MNHN D-T 1285: (A) longitudinal section through the body; (B) principal spicule; (C) intermediary spicule; (D) small spicule; (E) and (F) exotyles, general view; (G) finely spined distal tip of the exotyle depicted in E, detailed view; (H) finely spined distal tip of the exotyle depicted in F, detailed view. Scale bars: A, 0.2 mm; B–F, 0.1 mm; G and H, 0.05 mm.
Original description: Trachyteleia stephensi Topsent, Reference Topsent1928, p. 152, pl. VI figure 11.
SYNONYMS AND CITATIONS
Trachyteleia stephensi (Boury-Esnault, Reference Boury-Esnault, Hooper and van Soest2002, p. 218, figure 15).
TYPE MATERIAL
MNHN D-T 1285 (slides from holotype), Island of Villafranca, Azores, NE Atlantic, 1740 m, Scientific campaigns of the Prince of Monaco, campaign in 1911, station 3150. Topsent based his description on a small sponge fragment which was completely used for preparations. We have examined his microscopy slides.
DESCRIPTION
External morphology
(according to Topsent, Reference Topsent1928)
Holotype was a piece of a cushion-shaped sponge. Its surface was hispid, grey in alcohol, without papillae.
Skeleton
Main choanosomal skeleton composed of radial tracts of principal spicules which cross the cortex (Figure 30A). Auxiliary choanosomal skeleton comprises free-scattered intermediary spicules. Cortex made of a superficial palisade of small spicules and an inner layer of criss-cross intermediary spicules, reinforced by exotyles protruding above the surface.
Spicules
(N = 13 for exotyles, N = 30 for other categories)
-
• Principal spicules – straight or more rarely gently curved, slightly fusiform tylostyles (Figure 30B). Length 337–508–602 µm, diameter of tyle 6.5–8.9–11.7 µm, proximal diameter of shaft 3.9–6.1–9.1 µm, maximum diameter of shaft 7.8–9.8–13.0 µm.
-
• Intermediary spicules resemble the principal tylostyles in shape (Figure 30C). Length 270–296–327 µm, diameter of tyle 3.9–5.3–7.8 µm, proximal diameter of shaft 1.3–2.9–5.2 µm, maximum diameter of shaft 3.9–6.3–10.4 µm.
-
• Small spicules – gently curved, slender tylostyles (Figure 30D). Length 184–223–265 µm, diameter of tyle 3.9–5.2–6.5 µm, proximal diameter of shaft 2.6–3.5–5.2 µm, maximum diameter of shaft 5.2–5.6–7.8 µm.
-
• Exotyles – fusiform tylostyles (Figure 30E, F). Length 653–712–770 µm, diameter of tyle 8.9–11.2–13.0 µm, proximal diameter of shaft 6.1–7.9–10.4 µm, maximum diameter of shaft 11.7–15.9–18.2 µm. Among the examined exotyles 10 had distal tips covered with tiny weakly developed spines (Figure 30G, H) and three were entirely smooth.
REMARKS
Trachyteleia stephensi has never been recorded again since it was described by Topsent (Reference Topsent1928). The record of this species among the demosponges from the Cape Verde Islands and tropical West Africa (Van Soest, Reference van Soest1993) is an obvious mistake (Van Soest, personal communication). Presence of tiny spines on the distal tips of exotyles is in fact the only distinguishing feature of Trachyteleia. This unstable feature seems to be insufficient evidence for the validity of this genus while other characters cannot be carefully examined on the poor material available.
Meanwhile, a number of other polymastiid species possess similar non-ornamented exotyles in addition to a standard set of two to three categories of monactines. Most of these species are currently allocated to Polymastia, e.g. P. invaginata, P. grimaldii Topsent, Reference Topsent1913 and P. hirsuta Kelly-Borges & Bergquist, Reference Kelly-Borges and Bergquist1997. But one of them, originally described as Tethea hispida Bowerbank, Reference Bowerbank1864, was placed in a separate genus, Suberitechinus, by de Laubenfels (Reference de Laubenfels1949). Boury-Esnault (Reference Boury-Esnault, Hooper and van Soest2002) recognized the validity of Suberitechinus hispidus as a species but synonymized Suberitechinus with Trachyteleia, although with some doubt. We have examined the slides prepared from the holotype of S. hispidus, BMNH 1868.8.27.18, and found several substantial differences between this species and T. stephensi. In S. hispidus the exotyles reach 4000 µm in length, several times longer than in T. stephensi. All observed exotyles of S. hispidus lack spines, while many principal spicules are polytylote, a feature not observed in T. stephensi. Thus, following Plotkin et al. (Reference Plotkin, Gerasimova and Rapp2012) we provisionally recognize both Trachyteleia and Suberitechinus as valid genera. However, detailed and comparative descriptions along with phylogenetic analyses based on molecular and other independent datasets on Suberitechinus and other polymastiids with non-ornamented exotyles are required for the definitive classification of these taxa.
Genus Tylexocladus Topsent, Reference Topsent1898
TYPE SPECIES
Tylexocladus joubini Topsent, Reference Topsent1898 (by original designation).
DIAGNOSIS
Thickly encrusting, spherical to hemispherical sponges, usually with a single exhalant papilla. Main choanosomal skeleton composed of radial tracts of principal monactines. Auxiliary choanosomal skeleton comprises free-scattered small monactines and may also include smooth centrotylote microxeas. All species with a superficial cortical palisade made either of small monactines reinforced by exotyles or exclusively of exotyles. Some species also with an inner cortical layer of criss-cross monactines. Principal and small monactines are usually tylostyles. Exotyles with denticulate distal ornaments and often with proximal tyles (cladotylostyles).
Tylexocladus hispidus Lévi, Reference Lévi and Crosnier1993
(Figure 31)

Fig. 31. Tylexocladus hispidus, holotype MNHN D-CL 3582: (A) habitus; (B) longitudinal section through the body, general view; (C) the same section, detail of central cortex; (D) the same section, detail of peripheral cortex; (E) principal tylostyle; (F) small tylostyle of central cortex; (G) small tylostyle of peripheral cortex; (H) small exotyles of peripheral cortex; (I) intermediary exotyles of central cortex. Scale bars: A, 2 mm; B, 1 mm; C and D, 0.2 mm; E–I, 0.1 mm.
Original description: Tylexocladus hispidus Lévi, Reference Lévi and Crosnier1993, p. 23, figure 6B.
SYNONYMS AND CITATIONS
Tylexocladus hispidus (Kelly-Borges & Bergquist, Reference Kelly-Borges and Bergquist1997, p. 396; Boury-Esnault, Reference Boury-Esnault, Hooper and van Soest2002, p. 207).
TYPE MATERIAL
Holotype: MNHN D-CL 3582 (specimen in alcohol), New Caledonia, SW Pacific, 20°34.35′S 166°53.90′E, 435 m, campaign BIOCAL on RV ‘Jean Charcot’ in 1985, station DW 08.
DESCRIPTION
External morphology
Holotype – cushion-shaped crust attached to sand grains, ~ 10 × 10 × 1 mm in size (Figure 31A). Surface whitish in colour, with sparse bristle of large exotyles and undercoat of slightly protruding smaller exotyles, without papillae.
Skeleton
Holotype lacks the major portion of its choanosome. Remnants of the choanosome comprise sparse radial tracts of principal spicules which fan and ascend to the cortex. Cortex better preserved. Major portion of the cortex comprises a dense palisade of small and intermediary exotyles crossed by a layer of criss-cross tylostyles in its medial zone and pierced by large exotyles ascending from the choanosome (Figure 31B). In a tiny spot of the surface without exotyles the ascending tracts of principal spicules form bouquets reinforced by sparse intermediary tylostyles. In the surrounding area the palisade is made of intermediary exotyles and the crossing layer comprising intermediary tylostyles is loose (Figure 31C). In the peripheral cortex the palisade is composed of small exotyles and crossed by a dense layer of small tylostyles (Figure 31D).
Spicules
(N = 7 for large exotyles, N = 10 for other categories)
-
• Principal spicules – usually straight, slender styles to subtylostyles. Length 450–557–610 µm, diameter of shaft 9.0–10.0–12.0 µm (Figure 31E).
-
• Intermediary tylostyles usually gently curved, slender (Figure 31F). Length 255–293–334 µm diameter of tyle 3.9–6.4–9.5 µm, diameter of shaft 3.4–8.5–12.1 µm.
-
• Small tylostyles curved, stout, occasionally fusiform (Figure 31G). Length 104–147–188 µm, diameter of tyle 4.7–9.8–13.0 µm, maximum diameter of shaft 3.3–9.2–12.4 µm.
-
• Small exotyles – stout club-shaped cladotylostyles (Figure 31H). Length 214–341–510 µm. Well-developed, smooth or occasionally tuberculated proximal tyles, 8.0–15.5–21.3 µm in diameter. Shafts expanding from 3.0–10.4–15.0 µm near proximal tyles to 6.0–21.5–28.8 µm at distal ends. Distal ends usually denticulate, with numerous acerated jags resembling the distal ornamentations of exotyles in Tylexocladus joubini. Some exotyles with bowl-like distal ornamentations formed by smooth jags fused together.
-
• Intermediary exotyles – fusiform cladotylostyles with weakly developed proximal tyles (Figure 31I). Length 800–967–1145 µm, maximum diameter of shaft 33.0–37.6–44.0 µm. Distal extremities looking like the acerated tips of ordinary monactines were cleft.
-
• Large exotyles – cladotylostyles resembling the intermediary exotyles in shape but appearing more slender. Length 3012–3876–4994 µm, maximum diameter of shaft 32.0–34.6–38.0 µm.
REMARKS
Tylexocladus hispidus differs from the type species of Tylexocladus, T. joubini, by the lack of a cortical palisade of small tylostyles and by the presence of three categories of cladotylostyles, the smallest resembling those of T. joubini and forming the peripheral palisade, the intermediary with narrowed and cleft distal extremities forming the central palisade and the largest resembling the intermediary ones in shape and making up the surface bristle. The lack of microxeas also discriminates T. hispidus from the type specimens of T. joubini, although some other specimens of the latter species lack the microxeas as well (see below).
Tylexocladus joubini Topsent, Reference Topsent1898
(Figures 32–34)

Fig. 32. Tylexocladus joubini, type and characteristic specimens: (A) lectotype MOM 04-0526a, habitus; (B) paralectotype MOM 04-0526b, habitus; (C) paralectotype MOM 04-0526c, habitus; (D) specimen MOM 04-1244a, habitus; (E) longitudinal section through the body of the lectotype, general view; (F) the same section, detail of cortex. Scale bars: A–D, 5 mm; E and F, 0.2 mm.
Original description: Tylexocladus joubini Topsent, Reference Topsent1898, p. 242, figure 2d.
SYNONYMS AND CITATIONS
Tylexocladus joubini (Topsent, Reference Topsent1904, p. 122, pl. I figure 9, pl. XII figures 10–11; Topsent, Reference Topsent1928 (part.): 151, pl. VI figure 4; Kelly-Borges & Bergquist, Reference Kelly-Borges and Bergquist1997: p. 395, figure 26A–B; Boury-Esnault, Reference Boury-Esnault, Hooper and van Soest2002, p. 207, figure 5).
Nec Tylexocladus joubini (Boury-Esnault et al., Reference Boury-Esnault, Pansini and Uriz1994, p. 75, figure 50).
TYPE MATERIAL
Lectotype (designated herein, see Figure 32A, the largest specimen from the sponges depicted by Topsent (Reference Topsent1904) in pl. I, figure 9): MOM 04-0526a (in alcohol), Azores, NE Atlantic, 39°21′20″N 33°26′08″W, 1360 m, Scientific campaigns of the Prince of Monaco, campaign in 1896 on yacht ‘Princesse Alice’, station 702.
Paralectotypes (Figure 32B–C): MOM 04-0526b-c (two specimens in alcohol), from the same sample as the lectotype.
Slides from the type series: MNHN D-T 853 (one slide), BMNH1930.7.1.22 (one slide).
COMPARATIVE MATERIAL EXAMINED
MOM 04-1244a-b (two specimens in alcohol), NE Atlantic, Azores, to the West from Florès, 1229 m, Scientific campaigns of the Prince of Monaco, campaign in 1905, station 2210. MNHN D-T 1242 (one slide): NE Atlantic, Azores, to the West from Florès, 914–650 m, Scientific campaigns of the Prince of Monaco, campaign in 1905, station 2214 (Topsent (Reference Topsent1928) recorded one intact specimen from this sample, but only a slide has been found).
DESCRIPTION
External morphology
Thickly encrusting sponges. Surface velvety to hispid, with single weakly developed exhalant papillae. Lectotype cushion-shaped, ~ 2 × 2 × 0.2 cm in size, with uniformly velvety surface (Figure 32A). Paralectotype MOM 04-0526b (Figure 32B) and specimen MOM 04-1244b (Figure 33A) with velvety surface bearing well-defined hispid marginal fringe. Paralectotype MOM 04-0526c (Figure 32C) uniformly hispid. Specimen MOM 04-1244a (Figure 32D) is a poorly preserved hispid fragment.

Fig. 33. Tylexocladus joubini, aberrant specimens: (A) specimen MOM 04-1244b, habitus; (B) specimen MOM 04-1244b, longitudinal section through the body; (C) specimen MOM 04-1244b, exotyle, general view; (D) proximal tyle of the exotyle depicted in C, detailed view; (E) artichoke-shaped distal extremity of the exotyle depicted in C, detailed view; (F) slide MNHN D-T 1242, polytylote exotyles (some with lateral processes). Scale bars: A and B, 2 mm, C, 0.1 mm; D and E, 0.02 mm; F, 0.1 mm.
Skeleton
Main choanosomal skeleton composed of radial tracts of principal spicules entering the cortex, radiating and expanding into bouquets (Figure 32E). In specimen MOM 04-1244b some of principal spicules protrude slightly above the cortex. Auxiliary choanosomal skeleton comprises free-scattered small tylostyles and microxeas (in most sponges studied) or only small tylostyles (in specimen MOM 04-1244b and on slide MNHN D-T 1242). Cortex (~ 190–200 µm thick) is a single palisade of small tylostyles, reinforced by exotyles (Figure 32F). In the lectotype and paralectotype MOM 04-0526c exotyles spread uniformly over the surface (Figure 32E, F). In paralectotype MOM 04-0526b and specimen MOM 04-1244b exotyles concentrated mainly at the periphery forming a marginal fringe (Figure 33B).
Spicules
(measurements based on four specimens, individual dimensions presented in Table 1, N = 8 for exotyles, N = 10 for other categories)
-
• Principal spicules – usually straight, slender tylostyles (Figure 34A–C). Length 550–930–1150 µm, diameter of tyle 12.2–14.6–18.2 µm, diameter of shaft 10.4–14.9–18.2 µm.
-
• Small spicules – stout, more rarely slender tylostyles (Figure 34D–G). Length 120–213–359 µm, diameter of tyle 6.5–8.4–11 µm, diameter of shaft 5.2–8.1–10.1 µm.
-
• Microxeas in type specimens and specimen MOM 04-1244a smooth, centrotylote (Figure 34H). Length (in type specimens) 65–91–115 µm, diameter of central tyle 3.0–4.4–5.5 µm. Not found in specimen MOM 04-1244b and on slide MNHN D-T 1242.
-
• Exotyles (cladotylostyles) in type specimens and specimen MOM 04-1244a straight, slightly fusiform (Figure 34I), with well-developed proximal tyles (Figure 34J) and denticulate distal ornamentations comprising numerous acerated jags (Figure 34K).
-
• Exotyles (cladotylostyles) in specimen MOM 04-1244b much larger than in type specimens (Table 1) and also distinguished by shape – they are usually bent at distal portions (Figure 33C) and possess well-developed proximal tyles (Figure 33D), nearly equidiametric shafts and prominent artichoke- or flowerbud-shaped distal knobs (Figure 33E).
-
• Exotyles (cladotylostyles) on slide MNHN D-T 1242 straight, stout, with well-developed proximal tyles, two–three ring swellings on shafts directly behind the tyles and denticulate distal ornamentations, occasionally with few extra distal swellings and/or lateral shoots (Figure 33F).

Fig. 34. Tylexocladus joubini, spicules of type specimens: (A) principal tylostyle, general view; (B) proximal tip of the tylostyle depicted in A, detailed view; (C) distal tip of the tylostyle depicted in A, detailed view; (D) small tylostyle, one of the largest in its category, general view; (E) proximal tip of the tylostyle depicted in D, detailed view; (F) distal tip of the tylostyle depicted in D, detailed view; (G) small tylostyle, one of the smallest in its caregory; (H) centrotylote microxea; (I) cladotylostyle, general view; (J) proximal tyle of the cladotylostyle depicted in I, detailed view; (K) denticulate distal extremity of the cladotylostyle depicted in I, detailed view. Tylostyles taken from paralectotype MOM 04-0526c, oxea and cladotylostyle taken from lectotype MOM 04-0526a. Scale bars: A, 0.1 mm; B and C, 0.01 mm; D, 0.1 mm; E and F, 0.01 mm; G, 0.1 mm; H, 0.01 mm; I, 0.1 mm; J and K, 0.01 mm.
Table 1. Individual spicule dimensions (in μm) for specimens of Tylexocladus joubini.
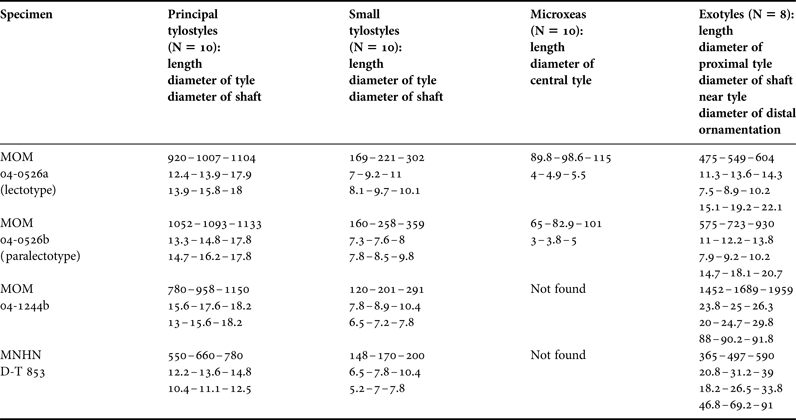
REMARKS
Except for the presence of cladotylostyles, T. joubini demonstrates many similarities with Atergia corticata Stephens, Reference Stephens1915 – external morphology, a single-layered cortex and choanosomal smooth microxeas (occasionally in Tylexocladus and characteristic of Atergia). These similarities led Topsent (Reference Topsent1928) to suggest that the presence of cladotylostyles was an unstable feature and he synonymized A. corticata with T. joubini. Among six Azorean specimens described as T. joubini in that paper by Topsent, four specimens (including MOM 04-1244a,b and MNHN D-T 1242 described above in the present paper) possessed cladotylostyles while two others lacked this category of spicules. We have examined one of the two sponges without exotyles, MOM 04-1244c. In addition to the principal and small tylostyles it has tylostyles of an extra category, 1700–2720 µm long, forming a marginal fringe which resembles the fringe of MOM 04-1244b made of cladotylostyles. The synonymy of A. corticata with T. joubini led to a number of misidentifications and confusion – Boury-Esnault et al. (Reference Boury-Esnault, Pansini and Uriz1994) recorded T. joubini without cladotylostyles from the Mediterranean, and Kelly-Borges & Bergquist (Reference Kelly-Borges and Bergquist1997) described a new species of Tylexocladus, T. villosus which also lacked cladotylostyles, from New Zealand. Evidenly, Tylexocladus and Atergia are closely affiliated genera, and only phylogenetic analyses based on molecular datasets can reveal the relationships between them. Here we follow Boury-Esnault (Reference Boury-Esnault, Hooper and van Soest2002) who proposed the allocation of all specimens with cladotylostyles to Tylexocladus regardless of whether they have microxeas or not, whereas all externally similar sponges possessing microxeas but lacking cladotylostyles are considered as Atergia.
Meanwhile, two non-type Azorean specimens with cladotylostyles differ from the type series by several features. Specimen MOM 04-1244b is distinguished by longer cladotylostyles with flowerbud-shaped distal knobs, while specimen MNHN D-T 1242 stands out for its polytylote cladotylostyles with occasional lateral shoots. Following Topsent (Reference Topsent1928) we regard these features as intraspecific variation. However, this assumption should be tested by more accurate molecular approaches on fresh material.
INCERTAE SEDIS
Polymastia umbraculum Kelly-Borges & Bergquist, Reference Kelly-Borges and Bergquist1997
(Figures 35 & 36)

Fig. 35. Polymastia umbraculum, holotype NZNM Por 66: (A) longitudinal section through the body, general view; (B) the same section, detail of cortex; (C) principal strongyloxeas; (D) intermediary subtylostyles; (E) slender small styles; (F) stout small tylostyles; (G) centrotylote oxea; (H) oxea lacking tyle. Scale bars: A, 3 mm; B, 0.2 mm; C, 0.1 mm; D, 0.05 mm; E–H, 0.01 mm.

Fig. 36. Polymastia umbraculum, holotype NZNM Por 66, exotyles: (A) exotyle without distal knob, general view; (B) proximal tip of the exotyle depicted in A, detailed view; (C) tuberculated distal extremity of the exotyle depicted in A, detailed view; (D) exotyle with distal knob, general view; (E) proximal tip of the exotyle depicted in D, detailed view; (F) umbrelliform distal knob of the exotyle depicted in D, detailed view. Scale bars: A, 0.1 mm; B, 0.001 mm; C, 0.001 mm; D, 0.1 mm; E, 0.001 mm; F, 0.001 mm.
Original description: Polymastia umbraculum Kelly-Borges & Bergquist, Reference Kelly-Borges and Bergquist1997, p. 380, Figure 12.
TYPE MATERIAL
Holotype (specimen in alcohol, a fragment studied): NZNM Por 66, Vivian Bay, Kawau Island, Hauraki Gulf, New Zealand, 36°25′S 174°51′E, 6 m, 10.02.1990. Fragment of holotype (studied): BMNH 1996.2.22.7.
Paratypes (several specimens, not studied): NZNM Por 549, from the same locality as the holotype, 02.01.1990.
DESCRIPTION
External morphology
(according to Kelly-Borges & Bergquist, Reference Kelly-Borges and Bergquist1997)
Encrusting sponges growing in oblong patches, 6–7 × 3–4 cm wide and ~ 2 cm thick. Surface granular with foraminiferan symbionts and microhispid with projecting spicules. Papillae considerably reduced. Colouration in life – surface yellowish orange, interior dark orange.
Skeleton
(our observations)
Main choanosomal skeleton composed of tracts of principal spicules. Few thicker tracts (120–250 µm thick) run longitudinally and form numerous thinner meanders in both horizontal and vertical direction, making up a network (Figure 35A). This network reinforced by auxiliary choanosomal skeleton of small and intermediary spicules. Numerous foraminiferans spread over the choanosome. Kelly-Borges & Bergquist (Reference Kelly-Borges and Bergquist1997) recorded choanosomal stellate crystal formations, but we have not observed such structures. Cortex with a superficial palisade composed of bouquets of small spicules (Figure 35B) reinforced by exotyles forming a thin hispidation above and supported by wide fanned brushes of intermediary spicules from below. Irregularly arranged criss-cross intermediary spicules build an inner cortical layer. Single intermediary spicules and occasional smooth microxeas overlay the superficial palisade and the middle layer of spicule brushes. Symbiotic foraminiferans and crustaceans embedded in the cortex.
Spicules
(our observations, N = 13 for exotyles, N = 10 for other categories)
-
• Principal spicules – strongyloxeas to fusiform subtylostyles, often polytylote (Figure 35C). Length 573–606–668 µm, maximum diameter of shaft 9.2–10.2–11.3 µm.
-
• Intermediary spicules – gently curved styles to subtylostyles (Figure 35D). Length 343–428–479 µm, maximum diameter of shaft 4.9–8.1–9.8 µm.
-
• Small spicules of two subcategories – (1) Slender styles with stepped distal tips (Figure 35E). Length 49–69–103 µm, diameter of shaft 1.0–1.2–1.5 µm. (2) Stouter tylotyles to subtylostyles (Figure 35F). Length 102–145–212 µm, diameter of tyle 3.3–4.5–6.4 µm, proximal diameter of shaft 1.7–3.0–4.7 µm, maximum diameter of shaft 1.9–4.0–6.6 µm.
-
• Smooth microxeas centrotylote (Figure 35G) or without tyles (Figure 35H). Length 79–176–215 µm, central diameter 1.5–1.9–3.0 µm.
-
• Exotyles filiform, flexous (Figure 36A, D). Length 167–441–552 µm, diameter of shaft 1.0–2.4–4.1 µm. Proximal extremities rounded, occasionally with weakly developed tyles (Figure 36B, E). Distal extremities of irregular shape, varying from slightly tuberculated tips (Figure 36C) to clubbed knobs, occasionally umbrelliform knobs with weakly developed protuberances resembling the distal ornamentations on the exotyles in Proteleia sollasi (Figure 36F).
REMARKS
The allocation of this species to a particular genus is difficult as it demonstrates affinities with several genera. The extremely thin exotyles with irregular, clubbed or occasionally umbrelliform distal extremeties resemble those in Proteleia sollasi, the reticulated choanosomal skeleton is similar to that in Weberella spp. and the smooth centrotylote microxeas recall the microxeas in Tylexocladus joubini and Atergia corticata. The reduced papillae of P. umbraculum are reminiscent of some suberitids rather than polymastiids. As none of these features are present in the type species of Polymastia, P. mamillaris, and P. umbraculum does not fit well into any other existing genus, awaiting evidence from molecular studies, we propose to keep it as incertae sedis.
DISCUSSION
Discrimination between the polymastiid genera and species with exotyles was for years mainly based on the shape of distal ornamentations of these spicules (Ridley & Dendy, Reference Ridley and Dendy1886, Reference Ridley and Dendy1887; Swarczewsky, Reference Swarczewsky1906; Topsent, Reference Topsent1898, Reference Topsent1928; Koltun, Reference Koltun and Bogorov1970; Boury-Esnault, Reference Boury-Esnault, Hooper and van Soest2002). Our study has shown the significance of other characters classified in six groups – (1) number and prominence of papillae, (2) presence of a surface hispidation formed by the protruding tracts of principal spicules, (3) architecture of cortex, (4) density of exotyles in the cortex, (5) size of principal spicules and exotyles, (6) presence of extra spicule categories in addition to the ordinary ones (Table 2). Affinities of the species presented above can therefore be reconsidered in view of these characters.
Table 2. Discriminating characters of polymastiid species with ornamented exotyles.

Six species of Sphaerotylus including the type species, S. capitatus, along with S. exotylotus, S. raphidophora, S. sceptrum, S. vanhoeffeni and S. verenae share the presence of weakly developed papillae, relatively short (less than 2 mm) principal spicules, and a delicate but dense surface echination formed by numerous protruding exotyles (Table 2). These exotyles are relatively short (less than 2 mm) and stout, with distal extremities bearing regular ornamentations which vary from weakly developed (S. raphidophora, S. sceptrum, S. vanhoeffeni and S. verenae) to well-developed spherical or subspherical knobs (S. capitatus and S. exotylotus). Architecture of the cortex in these six species varies greatly. It may comprise a single palisade of exotyles (S. exotylotus and S. vanhoeffeni), a single palisade of small tylostyles (S. raphidophora) or a superficial palisade of small tylostyles together with an inner layer of criss-cross intermediary monactines delimited by a zone with few spicules (S. capitatus). In S. sceptrum and S. verenae the architecture of the cortex in the areas around papillae and in the periphery is different. Of the six species considered above, five species possess extra spicule categories in addition to ordinary monactines in their auxiliary choanosomal skeleton – exotyles in S. capitatus, S. exotylotus, S. sceptrum and S. vanhoeffeni and raphides in trichodragmata in S. raphidophora.
The type specimens of the type species of Tylexocladus, T. joubini, possess at least three affinities with Sphaerotylus raphidophora – the presence of weakly developed papillae, a single-layered cortex comprising just a palisade of tylostyles and a delicate but dense superficial echination formed by numerous short and stout exotyles (Table 2). The distinguishing features of these specimens of T. joubini are the presence of centrotylote microxeas in the choanosome and the presence of expanded denticulate distal ornamentations on the exotyles. Unlike the type specimens, an aberrant specimen of T. joubini, MOM 04-1244b, lacks microxeas and possesses a heterogeneous surface with a central area free of exotyles and a marginal zone echinated by long (more than 2 mm) exotyles bearing artichoke-shaped distal ornamentations (Table 2). The other species of Tylexocladus, T. hispidus, is distinguished by a cortex comprising a palisade made of short exotyles of two categories, intermingled with a layer of criss-cross small tylostyles and reinforced by long (more than 2 mm) exotyles of third category forming a sparse surface hispidation (Table 2).
Four species including both species of Proteleia known so far, P. sollasi and P. tapetum, and two species of Sphaerotylus, S. isidis and S. strobilis, share the presence of well-developed papillae, relatively short (less than 2 mm) principal spicules and a sparse surface echination formed either by both the tracts of principal spicules ascending from the choanosome and the exotyles (S. isidis and S. strobilis) or only by the exotyles (Proteleia spp.) (Table 2). The exotyles are relatively short (less than 2 mm), usually slender (even filiform in Proteleia spp.), with well-developed distal ornamentations which may be regularly spherical (S. isidis), strobile-shaped (S. strobilis), regularly umbrelliform or fungiform (P. tapetum) or of irregular, variable shape (P. sollasi). The cortex in these four species comprises at least two layers, a superficial palisade of small tylostyles and an inner layer of criss-cross intermediary monactines. In P. tapetum these layers are intermingled. In S. isidis and S. strobilis they are delimited by a zone with few spicules. In P. sollasi the superficial palisade and the inner layer are separated by an extra palisade of intermediary monactines.
Six species, namely four Sphaerotylus spp. (S. antarcticus, S. borealis, S. renoufi and S. tjalfei), the only species of Koltunia (K. burtoni), and the only species of Trachyteleia (T. stephensi), share the presence of a thick and dense surface hispidation formed by the tracts of principal spicules ascending from the choanosome and reinforced by exotyles (Table 2). A two-layered cortex comprising a superficial palisade of small tylostyles and an inner layer of criss-cross intermediary monactines is recorded in all these species except for K. burtoni. Well-developed papillae are shared by S. antarcticus, S. borealis and S. renoufi. Large principal spicules and exotyles often exceeding 2 mm in length are typical of K. burtoni, S. antarcticus and S. borealis, while in S. tjalfei only few spicules of these categories may reach such a length. Long exotyles are also occasionally present in S. renoufi. The shape of distal ornamentations on the exotyles varies greatly. In S. tjalfei the ornamentations are usually symmetrical spherical knobs. In S. antarcticus and S. borealis the ornamentations are variable, often irregularly umbrelliform or fungiform. A similar shape of the distal ornamentations is also observed in some exotyles in S. renoufi. In K. burtoni the ornamentations are grapnel-shaped, with conspicuous claws. In T. stephensi the exotyles are ordinary tylostyles with fine spines on the distal tips, and they are larger than the principal tylostyles.
Among the species studied, one, Polymastia umbraculum, is controversial with respect to its affinities to other genera (Table 2). Whilst its reduced papillae are reminiscent of some suberitids, the cortex, comprising a superficial palisade of small tylostyles underlain by two layers of intermediary monactines and reinforced by sparse filiform exotyles with minutely branching distal ornamentations resembles that of Proteleia spp. Finally, the reticulated choanosomal skeleton of P. umbraculum is similar to that in Weberella spp.
A look at the diversity of the polymastiids with ornamented exotyles from a biogeographic perspective reveals that the known distribution of the 14 species is limited to very small geographic areas. Among these, nine species are endemic to the Pacific. Four species of Sphaerotylus are widely distributed, and they comprise two pairs of morphological equivalents distributed in the polar and subpolar zones, each pair containing one species in the northern hemisphere and the other in the southern hemisphere. Substantial morphological and ecological similarities of S. borealis and S. antarcticus rouse a challenging hypothesis of the existence of a single species with a bipolar distribution (Koltun, Reference Koltun1976). Sphaerotylus capitatus and S. vanhoeffeni demonstrate more distinctions than revealed in the first pair, but still these species possess many affinities, and a careful re-examination of the Kerguelen and Namibian specimens assigned to S. capitatus (Boury-Esnault & Van Beveren, Reference Boury-Esnault and van Beveren1982; Uriz, Reference Uriz1988) can probably throw more light on their relationship.
Morphological affinities between the species addressed in the present study should be re-evaluated by an integrative phylogenetic approach based on comprehensive molecular and morphological datasets in order to reveal the natural relationships between all polymastiid species possessing exotyles, both ornamented and non-ornamented.
ACKNOWLEDGEMENTS
We would like to express our gratitude to all colleagues who kindly provided the access to the historical and recent sponge collections of their institutions, namely to Clare Valentine, Andrew Cabrinovic and Emma Sherlock (National History Museum, London), Bruce Marshall (Museum of New Zealand Te Papa Tongarewa, Wellington), Bernard E. Picton (Ulster Museum, Belfast), Carsten Lüter and Carsten Eckert (Museum für Naturkunde, Berlin), Michèle Bruni (Musée Océanographique de Monaco), Ole Secher Tendal (Natural History Museum of Denmark, University of Copenhagen), Boris Sirenko and Olga Sheiko (Zoological Institute of Russian Academy of Sciences, Saint-Petersburg), Claude Lévi and Isabelle Domart-Coulon (Muséum National d'Histoire Naturelle, Paris), Klaus Rützler (Smithsonian National Museum of Natural History, Washington), Nicole de Voogd and J. Koos van Egmond (National Museum of Natural History, Leiden). Egil Severin Erichsen (University of Bergen) and Marie-Louise Tritz (Naturmuseum Senckenberg, Frankfurt am Main) are acknowledged for their careful assistance at the SEM. Special thanks also to Dorte Janussen (Senckenberg Forschungsinstitutt und Naturmuseum, Frankfurt am Main) for giving access to her department laboratory where a substantial part of the histological work was done, Bernard E. Picton (Ulster Museum, Belfast), Natalia Chervyakova (Moscow State University) and Bjørn Tore Dragnes (OMNIMAR Dragnes, Tromsø) for providing fresh material and underwater pictures. Nicole Boury-Esnault (Aix Marseille Université, CNRS, IRD, Avignon Université, IMBE UMR), Michelle Kelly (National Centre for Aquatic Biodiversity and Biosecurity, National Institute of Water and Atmospheric Research, Auckland, New Zealand) and John N.A. Hooper (Queensland Museum, Australia) are thanked for their very useful comments on an earlier version of the manuscript. Thanks are also due to two anonymous reviewers for their helpful comments.
FINANCIAL SUPPORT
This study was supported by the Norwegian Biodiversity Information Centre, Artsdatabanken (grant to HTR, project number 70184219); the Norwegian Academy of Science and Letters (grant to HTR), Queen's University Belfast, School of Biological Sciences and the Beaufort Marine Biodiscovery Programme (grant to CM). The studentship of CM was also funded by the Beaufort Marine Biodiscovery Research Award under the Sea Change Strategy and the Strategy for Science Technology and Innovation (2006–2013), with the support of the Marine Institute, funded under the Marine Research Sub-Programme of the National Development Plan 2007–2013. The visit of AP to the Museum für Naturkunde in 2008 was financed by Deutsche Forschungsgemeinschaft (fellowship JA 1063/15-1), and the visit to the Natural History Museum in 2010 received support from the SYNTHESYS Project http://www.synthesys.info/ which was financed by European Community Research Infrastructure Action under the FP7 Integrating Activities Programme.










































