Introduction
Invertebrates in coral reefs represent a critical ecological role in biodiversity because they can be engaged in intricate relationships, impacting the reef integrity and structure (Glynn and Enochs, Reference Glynn, Enochs, Dubinsky and Stambler2011). To know these complex interactions and how they can affect the coral reefs, we need more information regarding invertebrates, including describing their diversity. Diversity in these ecosystems has been measured mostly with well-studied (e.g. corals) or visible macrofauna, but few studies address small invertebrates, which are harder to find, and whose taxonomy is less known (Plaisance et al., Reference Plaisance, Caley, Brainard and Knowlton2011). The biodiversity associated with coral reefs is usually miscalculated because numerous areas are poorly studied or sampled, and most of the reef species are cryptic, small-sized organisms living in holes or cracks, sometimes being nocturnal and/or well camouflaged in their habitat, then unnoticed (Glynn and Enochs, Reference Glynn, Enochs, Dubinsky and Stambler2011).
Cryptic species in coral reefs, such as sea slugs, represent an important portion of reef biodiversity (Plaisance et al., Reference Plaisance, Caley, Brainard and Knowlton2011; Jensen, Reference Jensen2013). Describing the diversity of sea slugs is difficult since their recording and identification require experience in collecting techniques, and a trained eye to locate them, as they are very small and well-camouflaged (Jensen, Reference Jensen2013; Goodheart et al., Reference Goodheart, Ellingson, Vital, Galvão Filho, McCarthy, Medrano, Bhave, García-Méndez, Jiménez, López, Hoover, Awbrey, De Jesus, Gowacki, Krug and Valdés2016). Worldwide, most of the recorded species of sea slugs are located in coral reefs of the Indo-Pacific Ocean, where it is estimated that 15–40% of the species are still undescribed (Gosliner et al., Reference Gosliner, Valdés and Behrens2018). In the Gulf of Mexico (GM) and the Caribbean Sea (CAR), circa 350 species are present (Valdés et al., Reference Valdés, Hamann, Behrens and DuPont2006; García and Bertsch, Reference García and Bertsch2009; Rosenberg et al., Reference Rosenberg, Moretzsohn, Garcia, Felder and Camp2009; Redfern, Reference Redfern2013); the Caribbean is considered one of the richest regions regarding sea slugs (García and Bertsch, Reference García and Bertsch2009). Many species complex have been described recently in the region and divided into new species for different groups of sea slugs (e.g. Ornelas-Gatdula et al., Reference Ornelas-Gatdula, Camacho-García, Schrödl, Padula, Hooker, Gosliner and Valdés2012; Krug et al., Reference Krug, Vendetti and Valdés2016; Ghanimi et al., Reference Ghanimi, Schrödl, Goddard, Ballesteros, Gosliner, Buske and Valdés2020; García-Méndez et al., Reference García-Méndez, Padula and Valdés2022). Records in the Mexican Atlantic coast are mostly from the southern GM (Campeche Bank) on the reefs: Cayo Arcas and Cayo Arenas (Ortigosa and Simões, Reference Ortigosa and Simões2019), Sisal, Madagascar and Serpiente (Ortigosa et al., Reference Ortigosa, Simões and Calado2013) and Alacranes (Sanvicente-Añorve et al., Reference Sanvicente-Añorve, Solís-Weiss, Ortigosa, Hermoso-Salazar and Lemus-Santana2012b; Ortigosa et al., Reference Ortigosa, Lemus-Santana and Simões2015); while in the Mexican CAR coast records are scattered in a few localities from new species publications, sea slug guides or books from the tropical northwestern Atlantic (Ortea and Espinosa, Reference Ortea and Espinosa1996; Valdés et al., Reference Valdés, Hamann, Behrens and DuPont2006; Ortea and Bacallado, Reference Ortea and Bacallado2007; Ortea et al., Reference Ortea, Moro, Bacallado and Caballer2014) and molluscan inventories for the Yucatan Peninsula (Vokes and Vokes, Reference Vokes and Vokes1983).
Two common collecting methods for this group of gastropods have been used: (1) a direct method, where a visual and careful search for sea slugs is made on suitable substrates preferred by these animals. This method is dependent on observation time and expertise. Also, (2) an indirect method that involves collecting all potential substrates where the slugs could be found and left in white trays until the level of oxygen decreases, and then, they are reviewed thoroughly to find cryptic as well as non-cryptic species (Goodheart et al., Reference Goodheart, Ellingson, Vital, Galvão Filho, McCarthy, Medrano, Bhave, García-Méndez, Jiménez, López, Hoover, Awbrey, De Jesus, Gowacki, Krug and Valdés2016). However, the diversity found with the latter method is strongly dependent on the type of substrate, and it implies potential damage to the ecosystem. An indirect method that could be an alternative for the study of the diversity of sea slugs is the Autonomous Reef Monitoring Structures (ARMS). These structures mimic reef complexity and are easy to manipulate and collect (Ransome et al., Reference Ransome, Geller, Timmers, Leray, Mahardini, Sembiring, Collins and Meyer2017); also, they are considered a non-invasive standardized sampling method enabling the comparison of results between different regions (Pearman et al., Reference Pearman, Chust, Aylagas, Villarino, Watson, Chenuil, Borja, Cahill, Carugati, Danovaro, David, Irigoien, Mendibil, Moncheva, Rodríguez-Ezpeleta, Uyarra and Carvalho2020).
ARMS have been implemented to estimate cryptic diversity in coral reefs (Zimmerman and Martin, Reference Zimmerman and Martin2004; Ip et al., Reference Ip, Chang, Oh, Quek, Chan, Bauman and Huang2022). They provide an ideal artificial substrate for the settlement of benthic groups, such as sponges, tunicates, bryozoans and algae (known substrates for sea slugs), and consequently, could increase the incidence of these gastropods (Palomino-Alvarez et al., Reference Palomino-Alvarez, Castillo-Cupul, Vital, Suárez-Mozo, Ortigosa, Paz-Ríos, Cervantes-Campero, Muciño-Reyes, Homá-Canché, Hernández-Díaz, Sotelo-Casas, Dávila-Jiménez, Hidalgo, García-González, Hernández-González, Tello-Musi, González-Muñoz, Ugalde, Rocha, Moreno-Mendoza, Guadarrama, Simões and Guerra-Castro2021a, Reference Palomino-Alvarez, Vital, Castillo-Cupul, Suárez-Mozo, Ugalde, Cervantes-Campero, Muciño-Reyes, Homá-Canché, Hernández-Díaz, Sotelo-Casas, García-González, Avendaño-Peláez, Hernández-González, Paz-Ríos, Lizaola-Guillermo, García-Venegas, Dávila-Jiménez, Ortigosa, Hidalgo, Tello-Musi, Rivera-Higueras, Moreno-Mendoza, Wicksten, Rocha, Vieira, Mendoza-Garfias, Simões and Guerra-Castro2021b). Nevertheless, this methodology has never been considered as an indirect approach to assess the diversity of this group of molluscs. Therefore, this research aimed to contribute to the southern GM and the Mexican CAR coral reefs’ sea slugs' diversity and distribution records using ARMS as an indirect, standardized and comparable collection method.
Materials and methods
A total of 58 ARMS were placed at a depth of 3–7 m for 1–2 years between October 2018 and July 2021 at Bajo de 10 reef in the southern GM (21°20′53.82″ N, 90°8′45.48″ W), La Bonanza reef in Puerto Morelos (20°57′53.54″ N, 86°48′52.194″ W) and Mahahual reef in the Mexican CAR (18°37′24″ N, −87°43′32″ W) (Figure 1). All sampling units were retrieved covering them with a 500 μm mesh underwater, bringing them to the surface and transported to the laboratory in individual boxes with aerated seawater for processing. Each ARMS was disassembled, and each plate was placed in a tray with seawater, where we searched exhaustively for sea slugs (Palomino-Alvarez et al., Reference Palomino-Alvarez, Castillo-Cupul, Vital, Suárez-Mozo, Ortigosa, Paz-Ríos, Cervantes-Campero, Muciño-Reyes, Homá-Canché, Hernández-Díaz, Sotelo-Casas, Dávila-Jiménez, Hidalgo, García-González, Hernández-González, Tello-Musi, González-Muñoz, Ugalde, Rocha, Moreno-Mendoza, Guadarrama, Simões and Guerra-Castro2021a).

Figure 1. Localities where ARMS were implemented in southern GM (Bajo de 10 = B10) and Mexican Caribbean (Puerto Morelos and Mahahual). Reef area data are from Burke and Maidens (Reference Burke and Maidens2004).
All sea slugs were photographed, when possible, and then anaesthetized in seawater with magnesium chloride (5–7%) for at least 1 h; later, they were preserved in glass containers with 70 or 96% ethanol. Specimens were deposited at Colección Regional de Moluscos de la Península de Yucatán (SEMARNAT: YUC-INV-240-01-11) in Facultad de Ciencias, UNAM, Sisal, Yucatan, Mexico. Species determination of live organisms was made with identification guides or recent literature (Valdés et al., Reference Valdés, Hamann, Behrens and DuPont2006; Redfern, Reference Redfern2013; Krug et al., Reference Krug, Vendetti and Valdés2016; Ghanimi et al., Reference Ghanimi, Schrödl, Goddard, Ballesteros, Gosliner, Buske and Valdés2020); nomenclature follows MolluscaBase eds. (2023), except for Fionidae (Cella et al., Reference Cella, Carmona, Ekimova, Chichvarkhin, Schepetov and Gosliner2016). Results include the number of organisms found, approximate maximum body length, localities where they were recorded, collection numbers, a brief description of the external morphology and remarks for all the undetermined species, and which need further confirmation. The distribution of all recorded species is shown in Table 1.
Table 1. Sea slug fauna found in ARMS in coral reefs from the GM (Bajo de 10) and the Caribbean (Puerto Morelos and Mahahual)

B10, Bajo de 10; PM, Puerto Morelos; MAH, Mahahual; CAR, Caribbean; GM, Gulf of Mexico.
The number of organisms found and locality is given. Month of collection for juvenile specimens is provided. Recorded distribution is given, except for undetermined species. New records of species are in bold, an asterisk (*) indicates if the new record is for the Mexican coast or a dagger (†) refers if the new record is for the GM, in the recorded distribution.
Results
A total of 242 organisms were found in 58 ARMS, belonging to 31 species; 20 were identified to the species level, while 11 were adult specimens determined up to genus or a higher taxonomic level. Juvenile organisms were found and were identified to a possible genus, representing seven potential additional species (Table 1). Nudibranchia was the group with the largest number of species recorded (58%), followed by Sacoglossa (22.6%), Cephalaspidea and Anaspidea (6.4%), and Pleurobranchida and Runcinida (3.2%); the juveniles were not considered in these percentages to reduce bias. More than half of the species (19) were found in Bajo de 10 (GM), while 15 were found in the Caribbean: 10 in Puerto Morelos and 5 in Mahahual. The only species simultaneously recorded in the three reefs was Elysia velutinus Pruvot-Fol, 1947. Even though Puerto Morelos and Mahahual belong to the same region, they only shared Elysia flava Verrill, 1901 and possibly Jorunna cf. spazzola (Er. Marcus, 1955), as potential juveniles of the species were found in both localities. The remaining species were recorded exclusively in one locality (Table 1).
Phylum MOLLUSCA Linnaeus, 1758
Class GASTROPODA Cuvier, 1795
Subclass HETEROBRANCHIA Burmeister, 1837
Order CEPHALASPIDEA P. Fischer, 1863
Family RETUSIDAE Thiele, 1925
Genus Retusa T. Brown, 1827
Retusa sp.
(Figure 2A, B)

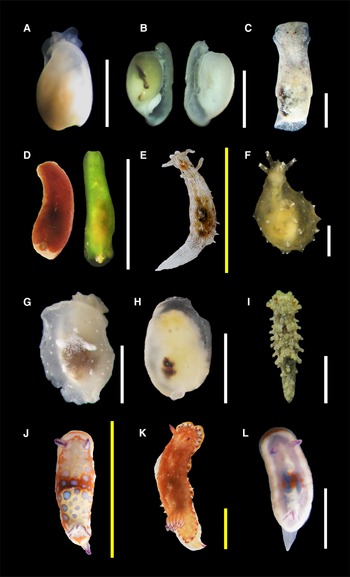
Figure 2. Species found in this study (authorities are given in Table 1): (A, B) Retusa sp.; (C) Haminoea elegans; (D) Lapinura sp.; (E) Stylocheilus polyomma; (F) Notarchus punctatus; (G) Berthella vialactea; (H) Berthella sp.; (I) Aegires cf. ortizi; (J) Felimida clenchi; (K) Felimida sp. 1; (L) Felimida sp. 2. Scale bars: A–D, F–I, L (white), 2 mm; E, J, K (yellow), 10 mm. H and L are considered juvenile specimens.
Material Examined
Ten organisms (3 mm), Bajo de 10 (CMPY-005728, CMPY-005740-41, CMPY-005755).
Description
Body colour translucent white with a dark area showing through the shell. Head with a cephalic shield, two lateral lobes and a frontal notch. Shell oval, wider in the centre with convex sides, of white colour; apex with a narrow umbilicus; aperture lip with a wing connected to the columellar margin; aperture has the length of the shell; sculpture reticulated, with spiral lines crossed by growth lines.
Remarks
Specimens resemble Retusa sp. 1 in Valdés et al. (Reference Valdés, Hamann, Behrens and DuPont2006) and Retusa sp. in Redfern (Reference Redfern2013).
Family HAMINOEIDAE Pilsbry, 1895
Genus Haminoea Turton & Kingston (in Carrington), 1830
Haminoea sp.
(photo not available)
Material Examined
One organism (3 mm), Bajo de 10 (CMPY-004455).
Description
Body colour opaque white. Shell translucent white, globose and fragile, with light spiral grooves crossed by fine axial growth lines; apex with a depression; wide aperture.
Remarks
Specimen similar to Haminoea sp. D in Redfern (Reference Redfern2013). Could be a juvenile specimen.
Order RUNCINIDA Burn, 1963
Family RUNCINIDAE H. Adams & A. Adams, 1854
Genus Lapinura Er. Marus & Ev. Marcus, 1970
Lapinura sp.
(Figure 2D)
Material Examined
Five organisms (2 mm), Puerto Morelos (CMPY-005770, CMPY-005838).
Description
Body elongated and narrow except in the centre which is slightly wider; background colour dark brown, the largest specimen (2 mm) had white iridescent blotches in the middle and margin of the notum; a green specimen was found with an orange tint in the posterior area of the notum (Figure 2D). Dorsum smooth. Posterior end of the body with a notch, and a small external translucent shell. In the green specimen, gill was visible in the notch and had at least four leaves, same coloration as the body. Eyespots visible.
Remarks
A Lapinura undescribed species of black colour had been previously recorded in Cozumel, Mexico by Valdés et al. (Reference Valdés, Hamann, Behrens and DuPont2006) as Runcina; no detailed description was provided, and it does not coincide with the specimens found in this study. Description and photographs of Lapinura divae in Redfern (Reference Redfern2013) resemble our specimens; however, the original description of this species does not mention any blotches or spots over the dorsum or in any part of the body (Ev. Marcus and Marcus, Reference Marcus and Marcus1963).
Order PLEUROBRANCHIDA
Family PLEUROBRANCHIDAE Gray, 1827
Genus Berthella Blainville, 1824
Berthella sp.
(Figure 2H)
Material Examined
One organism (3 mm), Mahahual (CMPY-004515).
Description
Body oval with an internal plate-like shell translucent white, very fragile; body colour translucent white. Rhinophores rolled, V-shaped. Gill on the right side of the body.
Remarks
This was a juvenile organism. Recently, Ghanimi et al. (Reference Ghanimi, Schrödl, Goddard, Ballesteros, Gosliner, Buske and Valdés2020) described two Berthella's species distributed in the Caribbean: Berthella vialactea and Berthella nebula, and mentioned some morphological differences that can help discern between them. However, the juvenile characteristics and the partial damage of this organism when it was found on the tray did not allow the identification to the species level.
Order NUDIBRANCHIA Cuvier, 1817
Family AEGIRIDAE P. Fischer, 1883
Genus Aegires Lovén, 1844
Aegires cf. ortizi Templado, Luque & Ortea, 1987
(Figure 2I)
Material Examined
One organism (6 mm), Puerto Morelos (CMPY-005771).
Description
Body elongated, broader at the centre and with a head relatively rounded; background colour greyish brown, covered with white dots and brown patches. Dorsum with many spiculose tubercles almost arranged in lines, and spicules visible throughout the body. Rhinophores smooth, of the same colour of the body, with a brown ring located ¼ before the distal end; each protected by three anterior tubercles. Gills covered by processes, with white tips. Eyespots visible in front of rhinophores at the base of tubercles.
Remarks
This specimen matched most of Aegires ortizi's description, including the structures protecting the rhinophores and gills, except by the distribution of tubercles and shape, as well as the shining white spots, which were both similar to those of Aegires gomezi (Ortea et al., Reference Ortea, Luque and Templado1990).
Family CHROMODORIDIDAE Bergh, 1981
Genus Felimida Ev. Marcus, 1971
Felimida sp. 1
(Figure 2K)
Material Examined
One organism (30 mm), Bajo de 10 (CMPY-005747).
Description
Body oval elongated; background colour orange with white spots, it has a white margin with purple dots and a yellow border. Dorsum soft with several irregular papillae, the largest located at each side of the body. Rhinophores orange with purple tips, and around 16 lamellae. Branchial sheath with 13 leaves, translucent orange and purple lines in the tip. Foot and ventral area white. Tail with a V shape, as wide as the body, of the same colour as the foot but with orange and purple tints.
Remarks
This species is similar to Felimida regalis; however, it presents a purple pattern in the gills and surrounding the mantle and has a different number of branchial leaves (Padula et al., Reference Padula, Bahia, Stöger, Camacho-García, Malaquias, Cervera and Schrödl2016).
Felimida sp. 2
(Figure 2L)
Material Examined
Two organisms (4 mm), Bajo de 10 (CMPY-005742).
Description
Body oval elongated; background colour white with light purple, it has a white margin bordered by a thin orange line that broadens in front of the head; a white band extends from in between the rhinophores to the base of the branchial sheath; right in the centre of the body, there is a blue patch and orange dots. Rhinophores with five visible lamellae, whitish at the base and with purple tips. At least three branchial leaves of the same colour of the body and purple lines. Eyespots visible behind rhinophores. Tail V-shaped, narrow, with the same translucent white colour of the body.
Remarks
This specimen was a juvenile of the Felimida clenchi-binza chromatic group. Colour pattern is not a reliable element to identify juvenile members of this group to the species level (Padula et al., Reference Padula, Bahia, Stöger, Camacho-García, Malaquias, Cervera and Schrödl2016). The smallest organism (<2 mm) did not have the gills developed yet.
Genus Felimare Ev. Marcus & Er. Marcus, 1967
Felimare sp. 1
(Figure 3B)

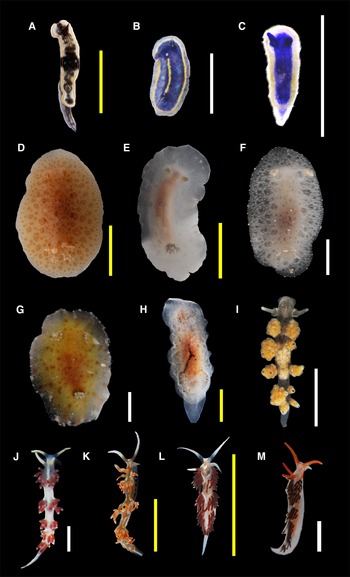
Figure 3. Species found in this study (authorities are given in Table 1): (A) Felimare ruthae; (B) Felimare sp. 1; (C) Felimare sp. 2; (D) Discodorididae sp.; (E) Jorunna cf. spazzola; (F) Jorunna cf. spazzola (juvenile); (G) Taringa telopia; (H) Hallaxa sp.; (I) Doto sp.; (J) Flabellina dushia; (K) Flabellina engeli; (L) Learchis sp. 1; (M) Learchis sp. 2. Scale bars: B, C, F, G, I, J, M (white), 2 mm; A, D, E, H, K, L (yellow), 10 mm. B, C, F and M are considered juvenile specimens.
Material Examined
Two organisms (2.5 mm), Bajo de 10 (CMPY-005745).
Description
Body slender; background colour blue with a whitish-transparent margin and an inner yellow margin, surrounding the blue area; a yellow line crosses the notum from head to tail in the centre. Ventral part of the body translucent white. Rhinophores with five lamellae of the same colour of the body, with an anterior yellow line and a posterior white line measuring half the length of the rhinophore. A yellow dot is almost located between the bases of rhinophores. With at least four branchial leaves of the same blue colour with yellow dots in the exterior area, forming a line. Four mantle dermal formations were visible in the ventral area of the mantle located at each side of the head and four in the posterior end.
Remarks
Organisms were juveniles from the blue chromatic group defined by Ortea et al. (Reference Ortea, Valdés and García-Gómez1996).
Felimare sp. 2
(Figure 3C)
Material Examined
One organism (1.3 mm), Puerto Morelos (CMPY-005773).
Description
Body slender; background colour blue with a broad white margin. A thin yellow line crosses most of the dorsum from head to tail in the centre and some yellow patches are present in the white margin. Ventral part of the body translucent white. Rhinophores smooth, of the same colour as the body. With branchial leaves of the same blue colour. We were not able to see mantle dermal formations.
Remarks
This was a juvenile from the blue chromatic group defined by Ortea et al. (Reference Ortea, Valdés and García-Gómez1996). Felimare sp. 2 is different from Felimare sp. 1 in the colour pattern of the body and the shape of rhinophores.
Family DISCODORIDIDAE Bergh, 1981
Discodorididae sp.
(Figure 3D)
Material Examined
One organism (27 mm), Mahahual (CMPY-005833).
Description
Body oval; background colour light brown, many darker brown dots on dorsum, most of them forming circles of different sizes, with the smallest in the centre and the border. Small caryophyllidia present, spicules visible throughout the body. Rhinophores of the same colour of the body, with a white tip and white dots making an anterior line, and around 20 lamellae. Six three-pinnate branchial leaves of the same colour of body, but with brown dots randomly distributed and white tips.
Remarks
This organism resembles Discodoridid sp. A from Redfern (Reference Redfern2013), but with a yellowish-brown colour.
Genus Jorunna Bergh, 1876
Jorunna cf. spazzola
(Figure 3E, F)
Material Examined
Five organisms (26 mm), Bajo de 10 (CMPY-005835), one organism (26 mm), Bajo de 10 (CMPY-005834); one organism (7.5 mm), Mahahual (CMPY-005842); one organism (7 mm), Puerto Morelos (CMPY-005777).
Description
Body oval; background colour white with grey circles and a light brownish area in the centre; margin with white widely spaced dots. Ventral area white with brown dots (except in juveniles) and a brown patch in the middle of the foot. Spicules visible throughout the body. Rhinophores light brown at the base and white tips, with at least five lamellae in juveniles (Mahahual and Puerto Morelos specimens) and at least 14 lamellae in adults (most of Bajo de 10 specimens). Gill with six retractile, bi-tripinnate branchial leaves of the same colour as mantle.
Remarks
These organisms resemble the description of J. cf. spazzola, which has been recorded in the Caribbean (Valdés et al., Reference Valdés, Hamann, Behrens and DuPont2006), and in the southern GM (Ortigosa et al., Reference Ortigosa, Simões and Calado2013). However, adults do not correspond with the maximum recorded size (9 mm) and have more lamellae in rhinophores (14) (Alvim and Pimenta, Reference Alvim and Pimenta2013). Also, juveniles of the Caribbean specimens from this work had a marked white-beige tip in rhinophores and branchial leaves that do not have the juveniles shown in Valdés et al. (Reference Valdés, Hamann, Behrens and DuPont2006). The mitochondrial cytochrome c oxidase I (COI) gene sequences were obtained from two specimens (CMPY-005834-35), but they did not match >90% any data in GenBank (National Center for Biotechnology Information, 2023).
Family ACTINOCYCLIDAE O'Donoghue, 1929
Genus Hallaxa Eliot, 1909
Hallaxa sp.
(Figure 3H)
Material Examined
One organism (35 mm), Bajo de 10 (CMPY-005739).
Description
Body oval elongated; background colour translucent white, with an orange patch in the centre of dorsum and brown dots scattered randomly; mantle with a brown-yellowish margin. Anterior part of the mantle is slightly wider. Dorsum soft with many papillae of different sizes, distributed throughout the notum. Ventral area and foot white with very small brownish dots. Rhinophores with 11–12 lamellae of the same colour as the body. Eight branchial bipinnate leaves. Tail wide and tongue-shaped.
Remarks
Most of Hallaxa spp. have been described for the Indo-Pacific Ocean; however, only one species is present in the Atlantic, Hallaxa apefae, described by Er. Marcus (Reference Marcus1957) and cited by Ortea and Buske (Reference Ortea and Buske2018). Descriptions from those publications did not match the organism found in this work, suggesting an undescribed species.
Family DOTIDAE Gray, 1853
Genus Doto Oken, 1815
Doto sp.
(Figure 3I)
Material Examined
One hundred and twelve organisms (4.5 mm), Bajo de 10 (CMPY-005730, 5733-35).
Description
Body slender with a narrow tail; background colour translucent white with black patches scattered in the entire body. Rhinophores smooth in a cup-like base, with black dots and some white patches, especially in the tips. Cerata grape-shaped with white dots and a characteristic black dot at the tip of most of the tubercles they had; digestive glands visible through the cerata and have different colours (orange, yellowish, white, grey). Most specimens had five pairs of cerata. Eyespots between rhinophores or in front of them. Foot of the same colour as the body.
Remarks
Organisms resemble Doto sp. D in Redfern (Reference Redfern2013).
Facelinidae Bergh, 1889
Genus Learchis Bergh, 1896
Learchis sp. 1
(Figure 3L)
Material Examined
Eight organisms (11 mm), Bajo de 10 (CMPY-005756).
Description
Body elongated with long and curved foot corners; background colour translucent white, white lines from the base of cerata draw an ‘x’ curved pattern in the dorsum; a white band with an orange line crosses the head and back, in between rhinophores and reaches the middle of oral tentacles, which are long and white, except in the base; an orange line is also present at each side of the head, between the base of tentacles and the first group of cerata. Rhinophores smooth, reddish-orange at the base and with a white distal half. Red cerata with white tips, long, slender and pointed; at least three groups of cerata are present. Long and thin tail of the same white colour of the foot.
Remarks
Specimens very similar to Learchis evelinae. However, the original description of this species does not mention the orange line between rhinophores (Edmunds and Just, Reference Edmunds and Just1983), and the tail of our specimens is longer and thinner (Redfern, Reference Redfern2013).
Learchis sp. 2
(Figure 3M)
Material Examined
Five organisms (8 mm), Bajo de 10 (CMPY-005724, 5726, 5753).
Description
Body elongated with short, curved foot corners, background colour translucent white; the head is white with orange oral tentacles, a line of the same orange colour is formed between oral tentacles and the base of tentacles and the first group of cerata; two blurred white lines are below the base of cerata. Rhinophores are smooth, of the same orange colour as oral tentacles. Cerata are slender and relatively short, brown with white tips; cerata are aligned and hard to count, two spaces between groups of them are notorious. Short and thin tail of white colour, the same as the foot.
Remarks
These organisms could be juveniles. As they had shorter oral tentacles, cerata, tail and distinct colour patterns from the smallest specimens of Learchis sp. 1, we recognize them as a different species.
Family FIONIDAE Gray, 1857
Genus Tenellia A. Costa, 1866
Tenellia cf. tina (Er. Marcus, Reference Marcus1957)
(Figure 4B)

Figure 4. Species found in this study (authorities are given in Table 1): (A) Phidiana lynceus; (B) Tenellia cf. tina; (C) Tenellia sp. 1; (D) Tenellia sp. 2; (E) Berghia cf. creutzbergi; (F) Elysia crispata; (G) Elysia flava; (H) Elysia patina; (I) Elysia velutinus; (J) Elysia sp. 1; (K) Elysia sp. 2; (L) Polybranchia schmekelae; (M) Hermaea sp. Scale bars: B–H, J, M (white), 2 mm; A, I, K, L (yellow), 10 mm. J is considered a juvenile specimen.
Material Examined
One organism (2.5 mm), Bajo de 10 (CMPY-005718).
Description
Body slender with a long tail, almost half of the size of the body; background colour opaque white, with some shiny dots in the entire body; each oral tentacle has a white dot near the tip. A white glistening patch in the shape of W is in the dorsum, between the first group of cerata. Rhinophores smooth, with two white bands: the distal band is longer than the proximal band. Cerata elongated but relatively globose and pointed, light brown with white tips; there are two groups of cerata along the body. Eyespots in the base of rhinophores. Foot white transparent.
Remarks
Individual very similar to Tenellia tina. However, the coloration pattern of rhinophores, dorsum, cerata and oral tentacles do not fully correspond (Er. Marcus, Reference Marcus1957). Recently, Cella et al. (Reference Cella, Carmona, Ekimova, Chichvarkhin, Schepetov and Gosliner2016) studied the systematics of the family Tergipedidae, where Cuthona/Catriona tina was previously assigned. They found that this family is not monophyletic, and as it is part of a larger clade, they reassigned most of the members to Fionidae because it is the oldest name of the taxon. Also, the same authors considered that species previously recognized as Cuthona, should be changed to Tenellia (Cella et al., Reference Cella, Carmona, Ekimova, Chichvarkhin, Schepetov and Gosliner2016).
Tenellia sp. 1
(Figure 4C)
Material Examined
One organism (4.5 mm), Bajo de 10 (CMPY-005737).
Description
Body slender with a wide and short tail that has white dots; background colour opaque white with orange spots scattered in the dorsum; white patches in oral tentacles, and at each side of the body; a dashed white line at each side of the foot. A yellow line crosses the head in the centre and reaches the first group of cerata, as well as two parallel orange lines that start at the base of oral tentacles and continue at each side of the head; an orange line of the same colour is found dorsally between oral tentacles. Rhinophores smooth and white, with a brownish ring in the second proximal quarter. Cerata are relatively globose and pointed, light brown with white tips and a black base; presents at least four groups of cerata. Eyespots in the base of rhinophores. Foot of the same colour as the body.
Remarks
External morphology almost identical to Cuthona sp. recorded in Barbados by Edmunds and Just (Reference Edmunds and Just1983). See remarks of T. cf. tina for details about family and genus.
Tenellia sp. 2
(Figure 4D)
Material Examined
One organism (4.5 mm), Bajo de 10 (CMPY-005723).
Description
Body slender with a narrow tail; background colour opaque white; head with an orange area near the base of rhinophores. Rhinophores smooth, half white at the base and half red at the top. Cerata elongated and pointed, orange at the base, reddish at the top and with white tips; four groups of cerata are present. Eyespots at the base of rhinophores. Oral tentacles and foot of the same colour as the body.
Remarks
See remarks of T. cf. tina for details about family and genus.
Family AEOLIDIIDAE Gray, 1827
Genus Berghia Trinchese, 1877
Berghia cf. creutzbergi Er. Marcus & Ev. Marcus, 1970
(Figure 4E)
Material Examined
One organism (8 mm), Bajo de 10 (CMPY-005722).
Description
Body slender, long; background colour beige-whitish, with dense white pigmentation on the notum and head. Oral tentacles with a white dashed coloration, and a transparent base; presents foot corners almost triangular-shaped, with a rounded tip. Rhinophores covered by tubercles with slight brownish pigmentation, a white tip and a transparent base. Cerata globose, translucent white with notorious white rings in the middle of the cerata and a vanilla coloration at the tips and the base. Foot translucent white. Tail narrow, same colour as the foot.
Remarks
Specimen very similar to Berghia creutzbergi, but with a whiter coloration and more globose cerata; the white ring in the middle of cerata has not been mentioned in this species (Carmona et al., Reference Carmona, Pola, Gosliner and Cervera2014). Cerata move from side to side when crawling (Carmona et al., Reference Carmona, Pola, Gosliner and Cervera2014; Goodheart et al., Reference Goodheart, Ellingson, Vital, Galvão Filho, McCarthy, Medrano, Bhave, García-Méndez, Jiménez, López, Hoover, Awbrey, De Jesus, Gowacki, Krug and Valdés2016).
Superorder SACOGLOSSA
Family PLAKOBRANCHIDAE Gray, 1840
Genus Elysia Risso, 1818
Elysia sp. 1
(Figure 4J)
Material Examined
One organism (2 mm), Puerto Morelos (CMPY-005775).
Description
Body slender; background colour beige-white with green areas, especially around the head and in parapodia; two black-blue marks are visible on notum, below the white pericardium. Moustache in upper lip is present. Rhinophores rolled, of the same colour of body and dark-green in their anterior base. Parapodia smooth, without papillae, greenish with a beige glistening border. Eyespots behind the base of rhinophores. Foot translucent, with green digestive diverticula showing through.
Remarks
The single organism found was a juvenile and does not resemble any of the Elysia species from the Caribbean (Ortea et al., Reference Ortea, Moro, Bacallado and Caballer2014; Krug et al., Reference Krug, Vendetti and Valdés2016; Ortea, Reference Ortea2018).
Elysia sp. 2
(Figure 4K)
Material Examined
One organism (19 mm), Puerto Morelos (CMPY-005766).
Description
Body slender with tiny white papillae scattered in most of the body; background colour beige with olive-green and white-cream patches, as well as black and white spots. Rhinophores rolled, with white patches, an orange coloration and a black edge. Parapodia forming three siphonal openings when closed, they have the same colour of the body, a black margin and a submarginal orange band. Eyespots behind the base of rhinophores are hard to distinguish from black spots. Foot same colour as the body, with a green net of digestive diverticula showing through.
Remarks
This organism had some resemblance to Elysia ornata (Krug et al., Reference Krug, Vendetti and Valdés2016) but had tiny papillae and white-cream patches all over the body, among other differences mentioned in the description.
Family HERMAEIDAE H. Adams & A. Adams, 1854
Genus Hermaea Lóven, 1844
Hermaea sp.
(Figure 4M)
Material Examined
One organism (1.5 mm), Bajo de 10 (CMPY-005736).
Description
Body slender; background colour translucent white with red dots in the whole body and the red digestive system showing through; a regular pattern is observed in the dorsum. Rhinophores rolled, of the same body colour with the exception of two red lines going parallel from the proximal half of rhinophores, to each side of the head. Cerata elongated, transparent, with the vertical and red digestive glands visible, some of them were no longer embedded in the body. Eyespots behind the base of rhinophores. Foot of the same colour as the body.
Remarks
None of the described Hermaea species from the Caribbean resembles this organism regarding its characteristics or coloration pattern (Valdés et al., Reference Valdés, Hamann, Behrens and DuPont2006; Caballer-Gutiérrez and Ortea, Reference Caballer-Gutiérrez and Ortea2013).
Discussion
In this study, we found 31 species of heterobranch sea slugs using ARMS as an indirect collection method to study one locality from the southern GM and two from the Mexican CAR. This diversity represents 9.4% of the sea slug diversity recorded in the CAR (329 spp.) by García and Bertsch (Reference García and Bertsch2009). Even though some studies have addressed part of the study area (Valdés et al., Reference Valdés, Hamann, Behrens and DuPont2006; Ortigosa et al., Reference Ortigosa, Simões and Calado2013), here we report four determined species for the first time in the country. Further, B. cf. creutzbergi, Taringa telopia and T. cf. tina represent their first record in the GM. Also, Taringa and the family Actinocyclidae, represented in our study by Hallaxa sp., had not been previously recorded in the GM.
Most species were represented by only one specimen, limiting the identification to the species level. These numbers are frequently recorded for the group, as low abundances are found per species in the inventories in the CAR region (Valdés et al., Reference Valdés, Hamann, Behrens and DuPont2006; Ortigosa et al., Reference Ortigosa, Simões and Calado2013; Camacho-García et al., Reference Camacho-García, Pola, Carmona, Padula, Villani and Cervera2014; Goodheart et al., Reference Goodheart, Ellingson, Vital, Galvão Filho, McCarthy, Medrano, Bhave, García-Méndez, Jiménez, López, Hoover, Awbrey, De Jesus, Gowacki, Krug and Valdés2016) and the Campeche and Yucatan Bank (Sanvicente-Añorve et al., Reference Sanvicente-Añorve, Solís-Weiss, Ortigosa, Hermoso-Salazar and Lemus-Santana2012b; Ortigosa et al., Reference Ortigosa, Simões and Calado2013, Reference Ortigosa, Lemus-Santana and Simões2015; Ortigosa and Simões, Reference Ortigosa and Simões2019). Nevertheless, some species had high abundances in our samplings, such as Doto sp. (91 in one recovery event) or Phidiana lynceus (43 in eight recovery events), clearly demonstrating the potential of this collection method to study recruitment patterns, temporal population dynamics and substrate preferences of common and abundant sea slug species. Unlike the studies mentioned above, which used direct and indirect methods, here we assessed sea slugs' diversity exclusively by an indirect method and found similar results.
As with other indirect methods, where substrates are collected, we found juvenile specimens of small sizes, such as Felimida sp. and Felimare sp. However, we did not observe their adult state, which might have been searching for other suitable habitats. Very small individuals (<10 mm in total length as adults) and remarkably cryptic are usually found by indirect methods (Goodheart et al., Reference Goodheart, Ellingson, Vital, Galvão Filho, McCarthy, Medrano, Bhave, García-Méndez, Jiménez, López, Hoover, Awbrey, De Jesus, Gowacki, Krug and Valdés2016); ARMS proved their utility to find tiny organisms and could represent a methodological option for studying juvenile stages or life cycles of this group of molluscs.
Macroalgae is the most common substrate collected when studying sea slugs indirectly (Sanvicente-Añorve et al., Reference Sanvicente-Añorve, Hermoso-Salazar, Ortigosa and Solís-Weiss2012a). Then, herbivore groups are usually more abundant when using this method; species feeding on substrates attached to algae (e.g. hydroids and bryozoans) can also be present. In ARMS, several food sources for heterobranch sea slugs are found, such as sponges, ascidians, bryozoans, hydroids and algae, because the structures are used as a hard substrate base (Palomino-Alvarez et al., Reference Palomino-Alvarez, Castillo-Cupul, Vital, Suárez-Mozo, Ortigosa, Paz-Ríos, Cervantes-Campero, Muciño-Reyes, Homá-Canché, Hernández-Díaz, Sotelo-Casas, Dávila-Jiménez, Hidalgo, García-González, Hernández-González, Tello-Musi, González-Muñoz, Ugalde, Rocha, Moreno-Mendoza, Guadarrama, Simões and Guerra-Castro2021a, Reference Palomino-Alvarez, Vital, Castillo-Cupul, Suárez-Mozo, Ugalde, Cervantes-Campero, Muciño-Reyes, Homá-Canché, Hernández-Díaz, Sotelo-Casas, García-González, Avendaño-Peláez, Hernández-González, Paz-Ríos, Lizaola-Guillermo, García-Venegas, Dávila-Jiménez, Ortigosa, Hidalgo, Tello-Musi, Rivera-Higueras, Moreno-Mendoza, Wicksten, Rocha, Vieira, Mendoza-Garfias, Simões and Guerra-Castro2021b), thus increasing the possibility of finding specimens of different feeding habits.
Other studies in the area have documented 13–16% of undetermined species (Sanvicente-Añorve et al., Reference Sanvicente-Añorve, Hermoso-Salazar, Ortigosa and Solís-Weiss2012a, Reference Sanvicente-Añorve, Solís-Weiss, Ortigosa, Hermoso-Salazar and Lemus-Santana2012b). In contrast, in our study 47% were not identified at the species level. This result is related to the (1) juvenile condition, (2) lack of description of species previously recorded elsewhere and (3) potentially undescribed new species. The diversity of colour patterns at the juvenile stage in Felimida sp. 2, Felimare sp. 1, Felimare sp. 2 and Elysia sp. 1 is not reliable to provide a confirmation (Krug et al., Reference Krug, Vendetti and Valdés2016; Padula et al., Reference Padula, Bahia, Stöger, Camacho-García, Malaquias, Cervera and Schrödl2016). Also, five undetermined species from our research, including Retusa sp. and Doto sp., seem to have been formerly recorded in other localities but not described (Valdés et al., Reference Valdés, Hamann, Behrens and DuPont2006; Redfern, Reference Redfern2013). Having genetic data of the mentioned species could have helped in the identification, as molecular techniques are currently used to confirm species; however, the worldwide health situation due to COVID-19, along with our budget constraints, did not allow the sequencing of specimens. The only exceptions were two J. cf. spazzola specimens and one organism identified as T. telopia; their mitochondrial COI sequence was obtained before the mobility restrictions arrived at our country in early 2020. While J. cf. spazzola sequences did not match >90% any data in GenBank (National Center for Biotechnology Information, 2023), T. telopia's sequence coincided with the specimen recorded (accession no. MN720291) by Donohoo and Gosliner (Reference Donohoo and Gosliner2020).
Typically, the specific amounts of substrates collected in sea slugs' inventories using indirect methods are not mentioned. Most studies using the direct method report a great variation in the species richness and abundance (Thompson, Reference Thompson1976; Nybakken, Reference Nybakken1978; Sanvicente-Añorve et al., Reference Sanvicente-Añorve, Hermoso-Salazar, Ortigosa and Solís-Weiss2012a, Reference Sanvicente-Añorve, Solís-Weiss, Ortigosa, Hermoso-Salazar and Lemus-Santana2012b; Goodheart et al., Reference Goodheart, Ellingson, Vital, Galvão Filho, McCarthy, Medrano, Bhave, García-Méndez, Jiménez, López, Hoover, Awbrey, De Jesus, Gowacki, Krug and Valdés2016). For example, collecting effort (total searching time through direct observations for all observers) in the cited works consisted of 0.2–5.3 h invested for recording a species per locality (Goodheart et al., Reference Goodheart, Ellingson, Vital, Galvão Filho, McCarthy, Medrano, Bhave, García-Méndez, Jiménez, López, Hoover, Awbrey, De Jesus, Gowacki, Krug and Valdés2016). Even though collecting methods and their efforts are not comparable, it should be considered that a large underestimation in the species richness of the group remains a constant issue due to the strong dependence on sampling method and expertise (Nichols et al., Reference Nichols, Boulinier, Hines, Pollock and Sauer1998; Jensen, Reference Jensen2013). The new records on both coasts (southern GM and Mexican CAR), especially of genera and even family, stress the gap of information that we still need to fulfil in the area. Therefore, we recognize ARMS as a non-invasive useful sampling method to find juvenile, cryptic and rare species of sea slugs and other small or cryptic invertebrates, as well as to standardize their quantification and record their diversity.
Acknowledgements
We thank A. Hernández, P. Guadarrama, G. Martínez-Moreno and F. Mex from UMDI-Sisal, F. Ciencias, UNAM, Mexico for technical assistance on field trips and laboratory. E. Mex (Ziz Ha, Sisal) and M. Victoria (Dorado Buceo, Puerto Morelos) provided diving services which allowed the successful deployment and recovery of ARMS. We acknowledge Parque Nacional Arrecife de Puerto Morelos and M. C. García-Rivas. We thank D. Ugalde, T. García, P. Homa, R. Castillo, N. Suárez, R. Sotelo, R. Mendoza, G. Cervantes, M. Muciño, A. Pérez, P. Tapia, J. Rubio, O. Melo, M. García, A. Hernández, D. Espinosa, S. Santa-Cruz and C. Cruz for their support on field trips, logistics and sample processing. A. Valdés and P. Krug helped in the identification of some specimens. S. Santa-Cruz captured photographs of Elysia specimens (G, H, K) used in Figure 4. We thank J. L. Cervera-Currado and L. Carmona for DNA extraction and amplification, comprised at the Instituto Universitario de Estudios Marinos de la Universidad de Cádiz, Cadiz, Spain. X. G. V. (CVU: 564148) and L. A. P.-A. (CVU: 447073) acknowledge Consejo Nacional de Ciencia y Tecnología (CONACyT) for their PhD scholarships. X. G. V. acknowledge Posgrado en Ciencias Biológicas, UNAM and L. A. P.-A. acknowledge Posgrado en Ciencias del Mar y Limnología, UNAM. Samples were collected with permit No. PPF/DGOPA-082/19, issued by Secretaría de Agricultura, Ganadería, Desarrollo Rural, Pesca y Alimentación (SAGARPA). Collecting of ARMS and all specimens was allowed with permit No. EX.006/06/18 issued by Secretaría de Marina (SEMAR) of Mexico. This is a BDMY publication.
Author's contribution
L. A. P.-A., E. J. G.-C. and N. S. designed research. X. G. V. and L. A. P.-A. involved in material preparation and data collection. E. J. G.-C. and N. S. obtained funding for field trips and laboratory requirements. X. G. V. and D. O. analysed data. X. G. V., L. A. P.-A. and D. O. wrote the first draft of the manuscript and all authors commented on subsequent versions of the manuscript. All authors read and approved the final manuscript.
Financial support
Field and lab work was funded by grants to N. S. by the Harte Charitable Foundation through the Harte Research Institute for the Gulf of Mexico Studies, Texas A&M University at Corpus Christi and Comisión Nacional para el Conocimiento y Uso de la Biodiversidad (CONABIO-NE018).
Conflict of interest
The authors declare none.
Ethical standards
Collection and preservation of organisms were conducted according to all applicable institutional, local and international regulations.
Data
All data generated or analysed during this study are included in this published article. A dataset is available at Ocean Biodiversity Information Systems (https://doi.org/10.15468/kfzrq4). GenBank (https://www.ncbi.nlm.nih.gov/) accession number for Taringa telopia (CMPY-005840) is OQ606964; sequences from two organisms of Jorunna cf. spazzola (CMPY-005834-35) are available upon request.