Introduction
The Mediterranean Sea is considered a hotspot of biodiversity with a high rate of endemism (Bianchi & Morri, Reference Bianchi and Morri2000; Coll et al., Reference Coll, Piroddi, Steenbeek, Kaschner, Ben Rais Lasram, Aguzzi, Ballesteros, Bianchi, Corbera, Dailianis, Danovaro, Estrada, Froglia, Galil, Gasol, Gertwagen, Gil, Guilhaumon, Kesner-Reyes, Kitsos, Koukouras, Lampadariou, Laxamana, López-Fé de la Cuadra, Lotze, Martin, Mouillot, Oro, Raicevich, Rius-Barile, Saiz-Salinas, San Vicente, Somot, Templado, Turon, Vafidis, Villanueva and Voultsiadou2010). In recent decades climate change and heavy anthropic pressures are giving rise to important modifications in the rocky benthic biocoenoses of this semi-closed basin (Cerrano et al., Reference Cerrano, Bavestrello, Bianchi, Cattaneo-Vietti, Bava, Morganti, Morri, Picco, Sara, Schiaparelli, Siccardi and Sponga2000; Lejeusne et al., Reference Lejeusne, Chevaldonné, Pergent-Martini, Boudouresque and Pérez2010; Bianchi et al., Reference Bianchi, Morri, Chiantore, Montefalcone, Parravicini, Rovere and Stambler2012; Di Camillo & Cerrano, Reference Di Camillo and Cerrano2015; Montefalcone et al., Reference Montefalcone, De Falco, Nepote, Canessa, Bertolino, Bavestrello, Morri and Bianchi2018).
With almost 720 species (Pansini et al., Reference Pansini, Manconi and Pronzato2011; van Soest et al., Reference Van Soest, Boury-Esnault, Hooper, Rützler, de Voogd, Alvarez, Hajdu, Pisera, Manconi, Schönberg, Klautau, Picton, Kelly, Vacelet, Dohrmann, Díaz, Cárdenas, Carballo, Rios and Downey2018), sponges are one of the most diversified group of hard bottom organisms. The effects of global changes on the Mediterranean sponge fauna were approached in several papers evidencing different trajectories of changes. In some studies sponges, mainly keratosas, were among the groups most affected by the mass mortality events observed in the Mediterranean Sea during the last decades (Gaino & Pronzato, Reference Gaino and Pronzato1991; Gaino et al., Reference Gaino, Pronzato, Corriero and Buffa1992; Rizzello et al., Reference Rizzello, Corriero, Scalera-Liace and Pronzato1997; Pronzato, Reference Pronzato1999; Cerrano et al., Reference Cerrano, Bavestrello, Bianchi, Cattaneo-Vietti, Bava, Morganti, Morri, Picco, Sara, Schiaparelli, Siccardi and Sponga2000; Pronzato & Manconi, Reference Pronzato and Manconi2008; Garrabou et al., Reference Garrabou, Coma, Bensoussan, Bally, Chevaldonné, Cigliano, Diaz, Harmelin, Gambi, Kersting, Ledoux, Lejeusne, Linares, Marschal, Pérez, Ribes, Romano, Serrano, Teixido, Torrents, Zabala, Zuberer and Cerrano2009; Pronzato et al., Reference Pronzato, Ledda, Manconi, Lucas and Borja2012). Other studies demonstrated that some species enhanced their covering during the present episode of global warming. In the Ligurian Sea it was stated that the encrusting red sponge Crambe crambe (Schmidt, 1862) strongly increased covering in correspondence to the disease affecting the common anthozoan Parazoanthus axinellae (Schmidt, 1862) (Cerrano et al., Reference Cerrano, Totti, Sponga and Bavestrello2006). In the same area a survey on a coralligenous vertical cliff, 25 years after a previous one, indicated that some species, such as Axinella spp. and the red encrusting sponges (C. crambe – Spirastrella cunctatrix Schmidt, 1868), strongly increased their abundance while massive species such as Petrosia (Petrosia) ficiformis (Poiret, 1789) and Chondrosia reniformis Nardo, 1847 reduced their covering (Bertolino et al., Reference Bertolino, Betti, Bo, Cattaneo-Vietti, Pansini, Romero and Bavestrello2016). More recently an increase in sponge diversity was recorded in two Ligurian marine semi-submerged caves surveyed again 50 years after the first study (Costa et al., Reference Costa, Betti, Nepote, Cattaneo-Vietti, Pansini, Bavestrello and Bertolino2018). It thus is important to collect data about the trajectory of the change of different sponge communities from other regions of the Mediterranean Sea.
The coast of the Salento Peninsula (Apulia, south-east Italy) is characterized by karst cliffs rich in semi-submerged marine caves of environmental importance as emphasized by Sarà (Reference Sarà1974) and more recently by Belmonte et al. (Reference Belmonte, Costantini, Denitto, Della Tommasa, Miglietta, Onorato, Poto and Vetere1999) and Bussotti et al. (Reference Bussotti, Denitto, Guidetti and Belmonte2002, Reference Bussotti, Terlizzi, Fraschetti, Belmonte and Boero2006). A large area of coralligenous outcrops characterizes the continental shelf in front of this stretch of coast (Bracchi et al., Reference Bracchi, Savini, Basso, Marchese and Corselli2015, Reference Bracchi, Basso, Marchese, Corselli and Savini2017). This region, that accounts for more than 4% of the total Mediterranean sponge fauna (Sarà, Reference Sarà1960, Reference Sarà1969; Pulitzer-Finali, Reference Pulitzer-Finali1983; Corriero et al., Reference Corriero, Gherardi, Giangrande, Longo, Mercurio, Musco and Marzano2004; Longo et al., Reference Longo, Cardone, Mercurio, Nonnis Marzano, Pierri and Corriero2015, Reference Longo, Cardone, Pierri, Mercurio, Mucciolo, Marzano and Corriero2017) was thoroughly surveyed in the period 1967–70 by Pulitzer-Finali (Reference Pulitzer-Finali1983), particularly in the area of Tricase Porto (Otranto Strait). The rich Pulitzer-Finali reference collection (preserved at DiSTAV) of sponges offers a unique possibility to compare the present sponge fauna with that present in the same zone about 50 years ago, presumably before the onset of global warming.
The aim of this work is therefore the characterization of the sponge fauna living off Tricase Porto (Otranto Strait) and the comparison of the actual sponge fauna (2017) with that studied in the same area about 50 years ago. Special attention has been dedicated to the description of two new species recorded during the present survey.
Materials and methods
The study area is located off Tricase Porto, just south of the narrowest point of the Otranto Strait (Figure 1). The samples were collected between June and August 2017. The sampling methods were similar to those used by Pulitzer-Finali (Reference Pulitzer-Finali1983) in his faunistic collections conducted between 1967 and 1970. The focus was on coralligenous, semi-submerged caves, rocky substrate down to 40 m depth by scuba diving and deep waters to 60 m depth by trammel net and a dredge to collect coralligenous fragments.
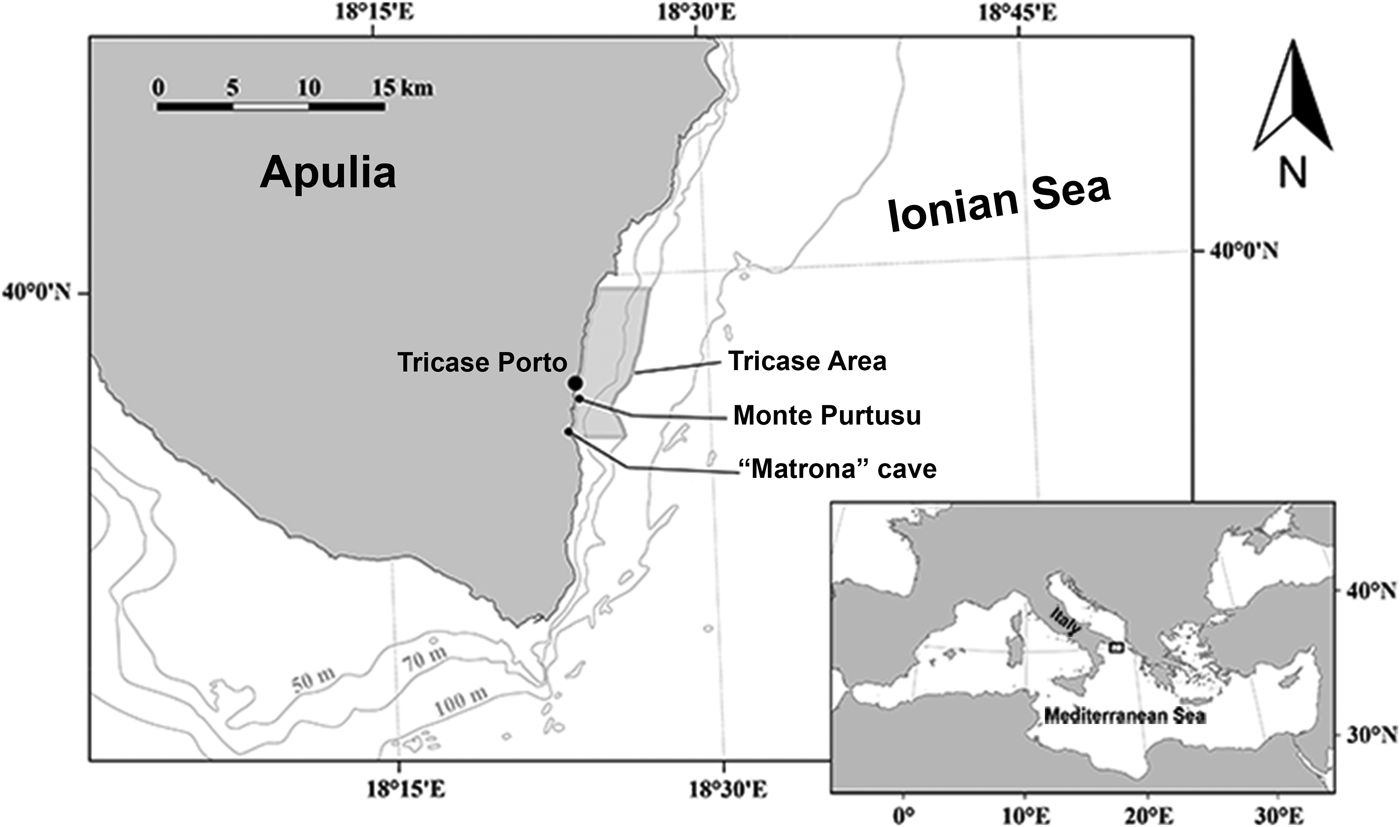
Fig. 1. Studied area in front of Tricase Porto (Otranto Canal – Ionian Sea).
For sampling we used the same techniques as Pulitzer-Finali (scuba diving and trammel), moreover we collected blocks of coralligenous formation by diving using hammer and chisel and we analysed them in the lab through the method described by Bertolino et al. (Reference Bertolino, Calcinai, Cattaneo-Vietti, Cerrano, Lafratta, Pansini, Pica and Bavestrello2014). Sponge samples were dried or preserved in 95% ethanol and processed by standard methods (Rützler, Reference Rützler, Stoddart and Johannes1978). Taxonomic decisions are in agreement with the Systema Porifera (Hooper & van Soest, Reference Hooper and van Soest2002), the Demosponge revision of Morrow & Cárdenas (Reference Morrow and Cárdenas2015) and the World Porifera Database (WPD) (van Soest et al., Reference Van Soest, Boury-Esnault, Hooper, Rützler, de Voogd, Alvarez, Hajdu, Pisera, Manconi, Schönberg, Klautau, Picton, Kelly, Vacelet, Dohrmann, Díaz, Cárdenas, Carballo, Rios and Downey2018). For the two new species length and width of at least 30 spicules per type were measured. Minimum, mean (in parentheses) and maximum values of spicule dimensions are reported. Dissociated spicules and dried tissues were transferred onto stubs, sputter coated with gold and observed by a scanning electron microscope (SEM). The type material of new species was deposited in the Museo Civico di Storia Naturale ‘G. Doria’ of Genova (MSNG). The type material of re-described species was deposited in the Department for Earth, Environment and Life Sciences (DiSTAV-University of Genoa).
The identified species were grouped according to their prevailing growth pattern, i.e. Massive/Erected (ME), Encrusting (En), Cavity dwelling (Cd) and Boring (Br).
Results
During the 2017 survey, a total of 110 sponge species (including two new species (Figures 2–3)) were recorded belonging to three classes: Calcarea (2), Homoscleromorpha (7) and Demospongiae (101) (Table 1). Twenty-five species are new findings for the Ionian Sea (Table 1; Table S1). Oceanapia perforata (Sarà, Reference Sarà1960) and Plakina reducta (Pulitzer-Finali, Reference Pulitzer-Finali1983) have been recorded for the second time after their original descriptions. Two species are new for science and are here described together with a detailed description of P. (S.) pulitzeri and P. (S.) vansoesti which cannot be distinguished according to their external morphology (Figures 4–6). Moreover, several specimens of Aplysina sp. recently recorded from a semi-submerged cave on the Ligurian coast (Costa et al., Reference Costa, Betti, Nepote, Cattaneo-Vietti, Pansini, Bavestrello and Bertolino2018) were recorded (Table 1). This species, living in the intertidal level, is composed of small cushion-shaped sponges, often connected by a net of stolons. The species is probably new but a genetic analysis is necessary for a definitive determination.
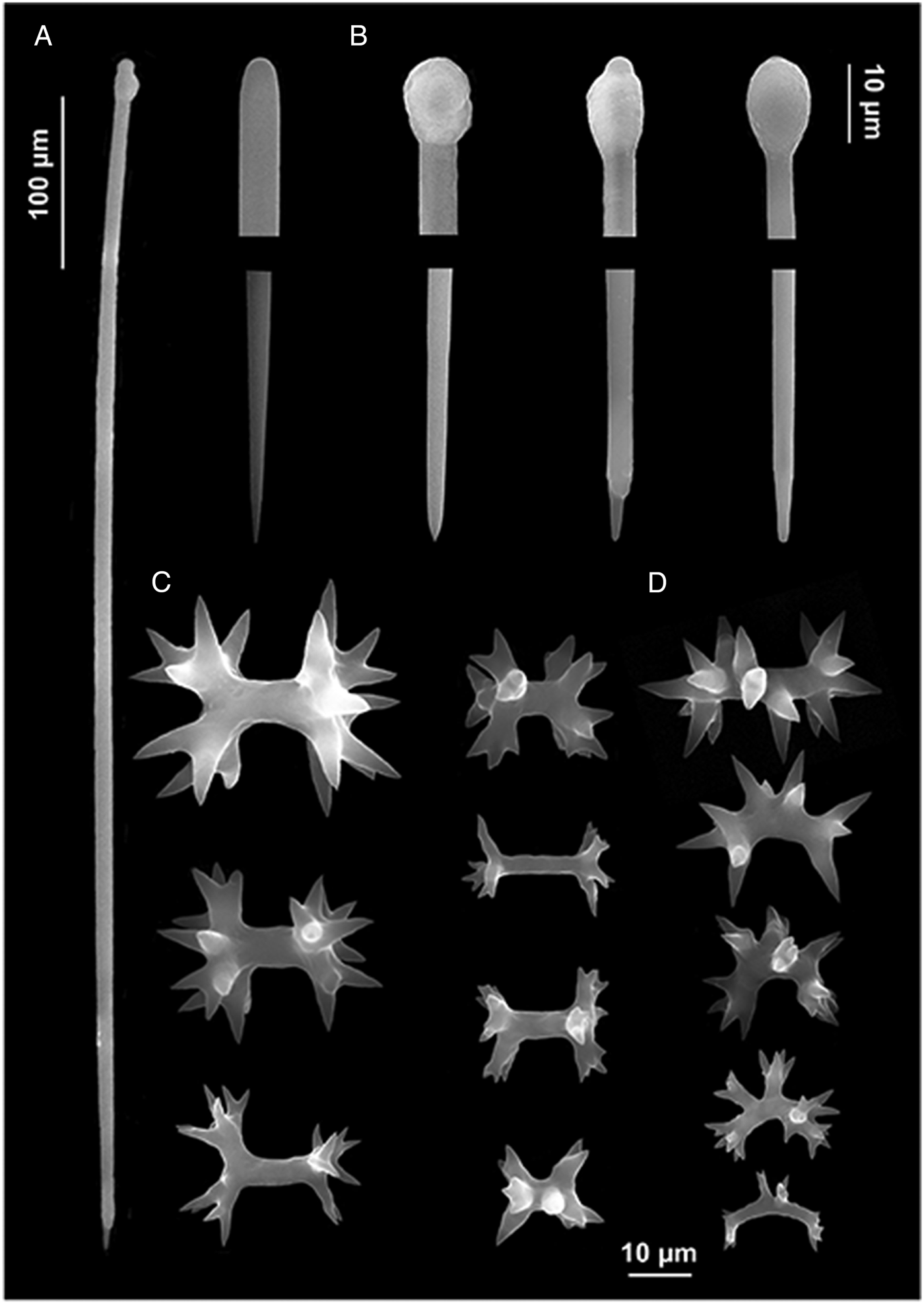
Fig. 2. Diplastrella boeroi sp. nov. (A) Long style; (B) style heads and tips; (C) diplasters to amphiasters; (D) spirasters.

Fig. 3. Spirastrella angulata sp. nov. (A) Long style; (B) style heads and tips; (C) spirasters.

Fig. 4. (A) P. (Strongylophora) pulitzeri; (B) P. (Strongylophora) vansoesti; (C) P. ficiformis with similar habit in the ‘Matrona’ cave at Tricase Porto.

Fig. 5. P. (Strongylophora) pulitzeri. (A) Ectosomal skeleton; (B) detail of a canal in the choanosomal skeleton; (C) choanosomal skeleton; (D) oxeas, strongyles and styles.
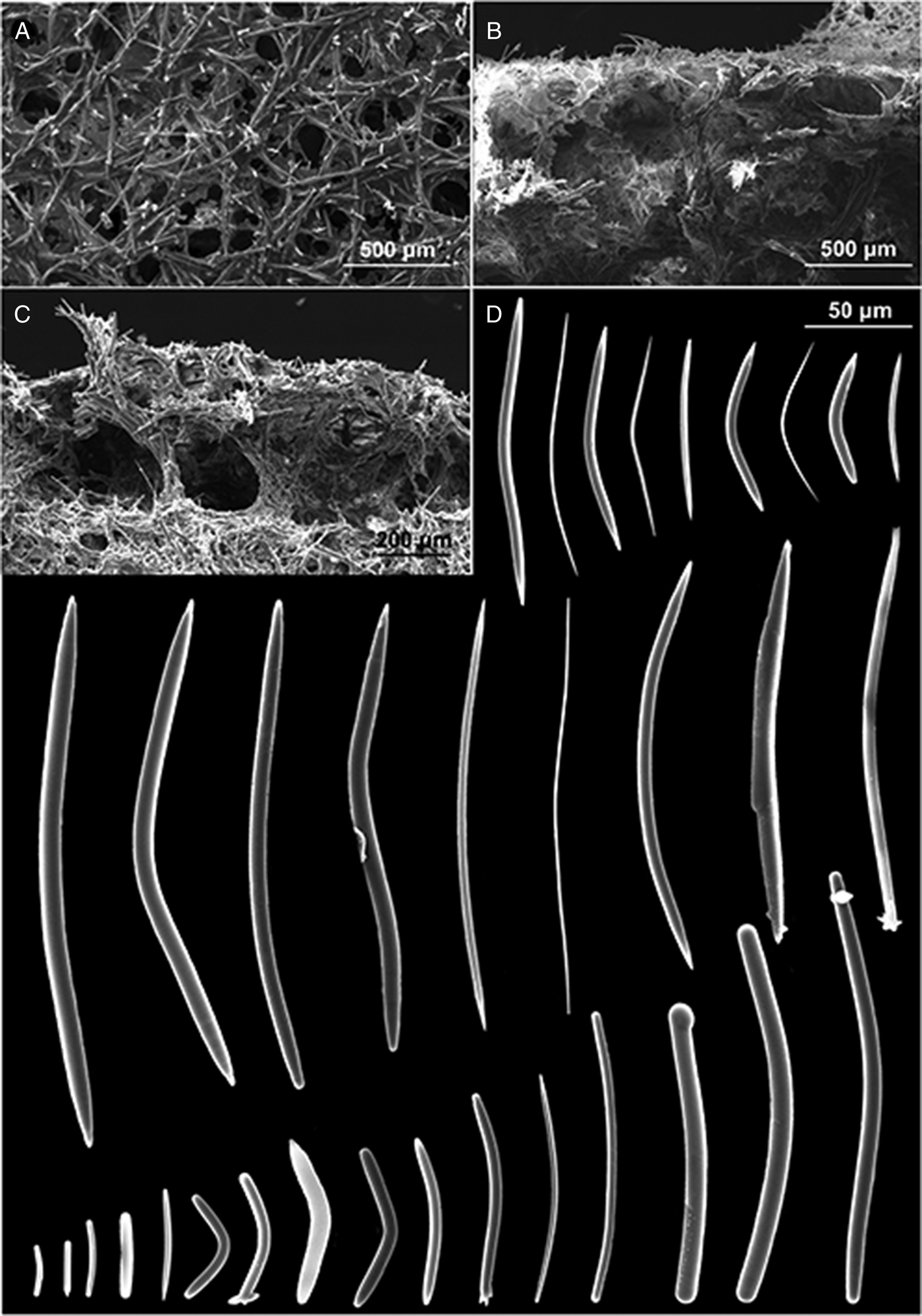
Fig. 6. P. (Strongylophora) vansoesti. (A) Ectosomal skeleton; (B) choanosomal skeleton; (C) detail of the choanosomal skeleton; (D) oxeas, strongyles and styles.
Table 1. List of sponge species collected at Tricase Porto sampled in the two periods with indication of their habit and type of habitat and the new finding for the Ionian Sea (*)

SYSTEMATICS
Class DEMOSPONGIAE Sollas, 1885
Subclass HETEROSCLEROMORPHA Cárdenas, Pérez & Boury-Esnault (Reference Cárdenas, Pérez, Boury-Esnault, Becerro, Uriz, Maldonado and Turon2012)
Order CLIONAIDA Morrow & Cárdenas, (Reference Morrow and Cárdenas2015)
Family SPIRASTRELLIDAE Ridley & Dendy, 1886
Genus Diplastrella Topsent, 1918
Diplastrella boeroi Bertolino, Costa & Pansini, sp. nov.
(Figure 2)
Type material
Holotype: (Specimens and slides) Tr.BL1_48 (MSNG 60884), Ionian Sea, Tricase Porto, (Otranto Strait) 39°55′32.1″N 18°24′00.7″E, depth 20 m, on a coralligenous concretion, 10.6.2017. Paratype: Tr.BL2_39. Adriatic Sea, Torre Guaceto, 40°42′57.51″N 17°48′12.61″E.
Other examined material: (Specimens and slides) Tr.BL1_F15_3A_351; Tr.BL1_F9B_273; Tr.BL2_39; Ionian Sea, Tricase Porto, (Otranto Strait) 39°55′32.1″N 18°24′00.7″E, depth 20 m, on a coralligenous concretion, 10.6.2017. TG.BL1_42; TG.BL1_F10A_14; Adriatic Sea, Torre Guaceto, 40°43′00.61″N 17°48′34.50″E, depth 24 m, on a coralligenous concretion, 12.6.2017.
Description
The specimens are small (about 2 cm in length) and thin (1–3 mm), encrusting on coralligenous concretions. The surface is slightly hispid. The colour in life is red and fades to orange after alcohol preservation. Live specimens are soft and show small elevated oscula.
Skeleton
Typical spirastrellidae structure consists of a base layer of spongin and microscleres. The tylostyles are erect, arranged in bouquets, with the heads embedded in the basal spongin layer. Some of them protrude through the sponge surface producing its hispidation. The ectosomal crust is mainly formed by diplasters.
Spicules
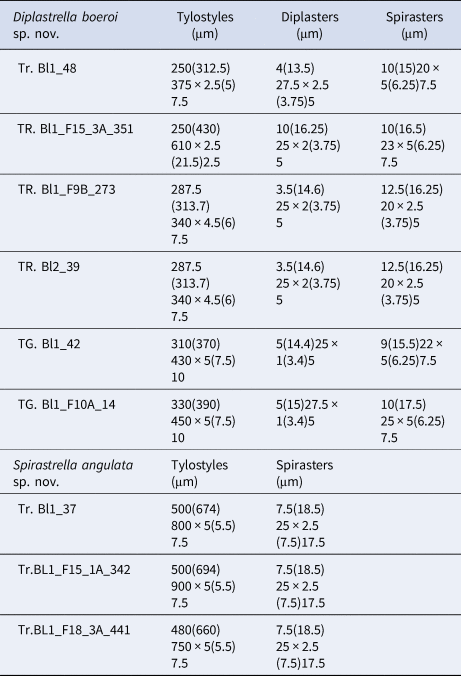
Megascleres: styles to tylostyles, straight or slightly curved, with circular or oval heads characterized by irregular or corrugate surface. Tips may be stepped, blunt, hastate and acerate (Figure 2A, B). They measure 250 (350) 450 × 2.5 (6.25) 10 µm. Microscleres: most spicules are diplasters with a smooth shaft and regular or sometimes irregular whirls of spines, 3.5 (15.5) 27.5 × 1 (3) 5 µm (Figure 2C). Spines are often bifid. Other microscleres are spirasters that sometimes show bouquets of spines on the shaft, 9 (17) 25 × 2.5 (5) 7.5 µm (Figure 2D) (Table 2).
Table 2. Spicule measures of specimens of Diplastrella boeroi sp. nov. and Spirastrella angulata sp. nov.
Etymology
The new species is named after Prof. Ferdinando Boero in recognition of his relevant contribution to biodiversity of the Salento coasts.
Habitat
Specimens recorded at 20–25 m depth encrusting the surface of coralligenous concretions and their superficial cavities.
Remarks
The species is assigned to the genus Diplastrella Topsent, 1918 according to the skeletal arrangement and the presence of diplasters. Seven species of the genus are known and two of them, D. bistellata (Schmidt, 1862) and D. ornata Rützler & Sarà, 1962, are recorded in the Mediterranean Sea. Both differ from the new species. D. bistellata has bigger microscleres (both spirasters and diplasters) than the new species: 45 µm in length vs 27.5 µm. D. ornata has large diplasters (56–76 µm) with characteristic multi-branched rays. The medium-sized ones may rarely assume the shape of thick spirasters but they do not represent a separate category of microscleres. In D. gardineri Topsent, 1918, a species with tropical distribution (Red Sea; Maldives; Zanzibar; Madagascar), the spirasters, 45 µm long, are much more numerous than the diplasters. D. megastellata Hechtel, 1965 and D. spiniglobata (Carter, 1879) have small asters and spheraster. D. spirastrelloides Van Soest, Reference Van Soest2017 from the North Atlantic has spirasters twice as large as the new species. D. yongmeoriensis Kim & Sim, Reference Kim and Sim2009 from the East China Sea has long and very thin spirasters similar to those found in the genus Cliona.
Genus Spirastrella Schmidt, 1868
Spirastrella angulata Bertolino, Costa & Pansini, sp. nov.
(Figure 3)
Type material
Holotype. (Specimens and slides) Tr.BL1_37, (MSNG 60885), Tricase Porto, 39°55′32.1″N 18°24′00.7″E, depth 20 m, on a coralligenous concretion, 10.6.2017.
Paratypes: (Specimens and slides) Tr.BL1_F15_1A_342; Tr.BL1_F18_3A_441, Tricase Porto, depth 20 m, on a coralligenous concretion, 10.6.2017, dry preserved.
Description
The three specimens were thinly encrusting (1–2 mm thick) on a coralligenous concretion, covering small surfaces (1–2 cm2). The colour in life is red and becomes orange in alcohol. The surface is slightly hispid. Live specimens are soft and show small elevated oscula.
Skeleton
The skeleton is typical of the genus Spirastrella with dense layers of spirasters in both ectosomal and basal choanosomal regions and bundles of a few tylostyles with the points directed outward. The smaller spirasters are concentrated at the surface, the larger ones close to the substrate, and many microscleres of both types are strewn in between. Tylostyles heads are embedded in the basal spongin layer. Some tylostyles protruding from the sponge surface cause a faint hispidation.
Spicules
Megascleres: Tylostyles to sub-tylostyles to styles, straight or curved and sometimes sinuous. Heads round to oval (sometimes showing malformations) and shafts with sharped points (Figure 3A, B). They measure 480 (690) 900 × 5 (5.5) 7.5 µm. Microscleres: all the microscleres are spirasters, 7.5 (18.5) 25 × 2.5 (7.5) 17.5 µm. The spiraster axis is generally twisted, angulate and sometimes curved and often once or twice bent (Figure 3C). The conical spines, always sharp, may be more or less evenly distributed or confined to the spicule extremities. A reduction in the number of spines may be observed in some of the spicules with a thin axis (Figure 3C) (Table 2).
Etymology
The species is named after the peculiar shape of the small angulate spirasters.
Habitat
Specimens were recorded at 20–25 m depth encrusting coralligenous concretions characterized by a pillar structure.
Remarks
Among the 17 known species of Spirastrella, only S. cunctatrix Schmidt, 1868 is until now recorded in the Mediterranean Sea and the Eastern Atlantic. S. cunctatrix differs from the new species for the morphology and the size of spicules. The tylostyles of S. angulata sp. nov. are remarkably longer than those of S. cunctatrix, whereas the spirasters of S. cunctatrix (28–50 µm in length) may be twice as big and different in shape from those of the new species. The two species present in the Red Sea: S. decumbens Ridley, 1884 and S. pachyspira Lévi, 1958, differ from S. angulata sp. nov. in the shape and size of spicules. S. decumbens has shorter tylostyles, S. pachyspira has very large spirasters up to 110 µm in length. The other known Spirastrella species have different characters in spicular complement (shape and measures) and live in geographically distant areas such as the Eastern Atlantic and the Caribbean, the Indian Ocean, the coast of Japan and a few Pacific areas.
Order HAPLOSCLERIDA Topsent, 1928
Family PETROSIIDAE van Soest, Reference Van Soest1980
Genus Petrosia Vosmaer, 1885
Subgenus Petrosia (Strongylophora) Dendy, Reference Dendy and Herdman1905
Petrosia (Strongylophora) pulitzeri Pansini, Reference Pansini1996
(Figures 4A–5)
Type material
(Specimens and slides) TRP64, Tricase Porto, Ionian Sea, semi-submerged ‘Matrona’ Cave (39°54′20.02″N 18°23′25.76″E), depth 2 m, 11.6.2017.
Description
Massive sponge with anastomosing horizontal irregular branches and with vertical irregular digitiform processes up to 1.5–2 cm high. Scattered round oscules are 1–5 mm wide. The surface is smooth with a cribrose aspect due to the presence of ostia (0.2–0.3 mm) (Figure 4A). Consistency is hard and colour is white in vivo and in alcohol preserved specimens.
Skeleton
The ectosomal skeleton is a network of irregular triangular or quadrangular meshes with sides formed by single strongyles or oxeas. At the angular points (‘corners’) of the strongyle-tracts are groups of very short strongyles which form surface nodes that produce the granular-aspect of the surface (Figure 5A). Choanosomal skeleton is a very dense, compact sub-rectangular network with small meshes formed by stout spicular tracts of closely packed strongyles and free spicules (Figure 5B, C). Toward the surface there are stout multispicular tracts formed by radiating bundles of closely packed strongyles, without visible spongin.
Spicules
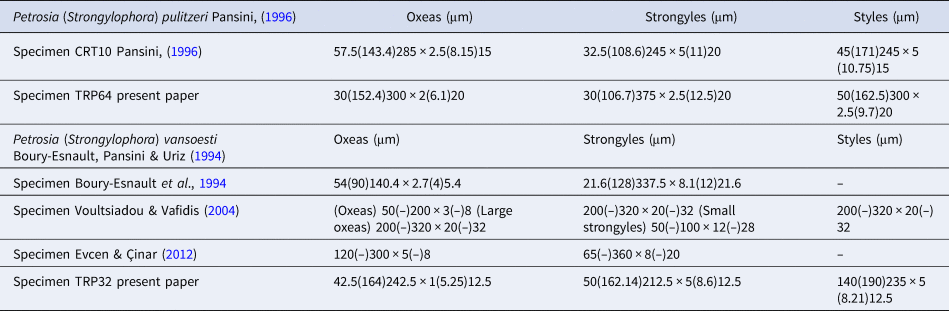
Megascleres: oxeas, measure 30 (152.4) 300 × 2 (6.1) 20 µm; styles measure 30 (106.7) 375 × 2.5 (12.5) 20 µm and strongyles with intermediate forms, measure 50 (162.5) 300 × 2.5 (9.7) 20 µm (Figure 5D).
Geographical distribution
Tyrrhenian Sea (Gulf of Naples), Aegean Sea (Crete), coasts of Turkey (Marmara Sea) (Pansini, Reference Pansini1996).
Petrosia (Strongylophora) vansoesti Boury-Esnault, Pansini & Uriz (Reference Boury-Esnault, Pansini and Uriz1994) (Figures 4B–6)
Type material
(Specimens and slides) TRP32, Tricase Porto, Ionian Sea, semi-submerged ‘Matrona’ Cave (39°54′20.02″N 18°23′25.76″E), depth 2 m, 11.6.2017.
Description
Massive sponge with external morphology similar to that of P. (Strongylophora) pulitzeri sampled by us (Figure 4B).
Skeleton
The ectosomal skeleton is a network of irregular triangular or quadrangular meshes with sides formed by single strongyles or oxeas (Figure 6A). At the angular points (‘corners’) of the strongyle-tracts are groups of very short strongyles which form surface nodes that produce the granular-aspect of the surface. Choanosomal skeleton very dense: compact sub-rectangular network of small meshes formed by stout tracts of closely packed strongyles and free spicules (Figure 6B, C). Toward the surface there are stout multispicular longitudinal tracts formed by radiating bundles of single strongyles, closely packed, without visible spongin.
Spicules
Megascleres: oxeas, measure 42.5 (164) 242.5 × 1 (5.25) 12.5 µm, styles, measure 50 (162.14) 212.5 × 5 (8.6) 12.5 µm and strongyles with intermediate forms, measure 14 (190) 235 × 5 (8.21) 12.5 µm (Figure 6D).
Geographic distribution
Saharan upwelling within the outflow of Mediterranean seawater in the Atlantic (Strait of Gibraltar), coast of Greece (Youra Island) and coast of Turkey.
Remarks
In the Matrona Cave, three species of the genus Petrosia (P. ficiformis, P. pulitzeri and P. vansoesti) were recorded. The external morphology does not allow their specific separation (Figure 4). While the spicules of P. ficiformis are well known, the other two species were less studied. In Table 3 we have compared data about morphological characters and ecology of P. (Strongylophora) pulitzeri Pansini, Reference Pansini1996 and P. (Strongylophora) vansoesti Boury-Esnault, Pansini & Uriz, Reference Boury-Esnault, Pansini and Uriz1994 specimens hitherto recorded in the Mediterranean Sea and in Table 4 we have compared data about the spicules of our specimens with those reported by previous description. Study of the skeleton (Figure 5A–C) of P. (Strongylophora) pulitzeri confirms that this species can be attributed to the subgenus Strongylophora as suggested by Lévi & Lévi (Reference Lévi and Lévi1983), de Weerdt & van Soest (Reference De Weerdt and van Soest1986) and Pansini (Reference Pansini1996). Petrosia (Strongylophora) pulitzeri is known for the Gulf of Naples, the Island of Crete (Pansini, Reference Pansini1996) and the Marmara Sea (Topaloğlu, Reference Topaloğlu2001). Petrosia (Strongylophora) vansoesti was described by Boury-Esnault et al. (Reference Boury-Esnault, Pansini and Uriz1994) from the Saharan upwelling within the outflow of Mediterranean seawater in the Atlantic (Strait of Gibraltar) and later recorded on the coast of Greece (Youra Island) by Voultsiadou & Vafidis (Reference Voultsiadou and Vafidis2004) and Turkey by Evcen & Cinar (Reference Evcen and Çinar2012).
Table 3. Morphological characters and ecology of P. (Strongylophora) pulitzeri Pansini, Reference Pansini1996 and P. (Strongylophora) vansoesti Boury-Esnault, Pansini & Uriz, Reference Boury-Esnault, Pansini and Uriz1994 specimens hitherto recorded in the Mediterranean Sea

Table 4. Spicule measurements of specimens of P. (Strongylophora) pulitzeri and P. (Strongylophora) vansoesti recorded in the Mediterranean Sea
Discussion and conclusion
In recent decades, marine ecosystem composition and functioning, especially in the Mediterranean Sea, have headed toward deep modifications driven by climate variations. It is well known that the biology of marine organisms can be altered by climate changes due to both a higher frequency of short-term extreme events and long-term temperature increases (Lejeusne et al., Reference Lejeusne, Chevaldonné, Pergent-Martini, Boudouresque and Pérez2010).
Long-term biodiversity monitoring and observations are essential for understanding the changes over time of the marine benthic communities (Roberts, Reference Roberts2009; Boero et al., Reference Boero, Kraberg, Krause and Wiltshire2015). In this context, sponges, due to their high biodiversity and long persistence of their spicular remains, are a suitable group to check putative differences occurring over a number of temporal scales (Bertolino et al., Reference Bertolino, Betti, Bo, Cattaneo-Vietti, Pansini, Romero and Bavestrello2016, Reference Bertolino, Costa, Carella, Cattaneo-Vietti, Cerrano, Pansini, Quarta, Calcagnile and Bavestrello2017a, Reference Bertolino, Cattaneo-Vietti, Costa, Pansini, Fraschetti and Bavestrello2017b; Costa et al., Reference Costa, Betti, Nepote, Cattaneo-Vietti, Pansini, Bavestrello and Bertolino2018). Thanks to the data of Pulitzer-Finali (Reference Pulitzer-Finali1983) recorded about 50 years before the present survey, a first comparative investigation of the sponge assemblages present in four habitats of Tricase Porto coastal area was possible.
The number of sponge species recorded in the Tricase area was about twice that observed 50 years before in the same stretch of coast (Table 1 and Figure 7). Thirty-one species (62% of the species recorded in the period 1967–70) are shared by the two surveys (Figure 7).
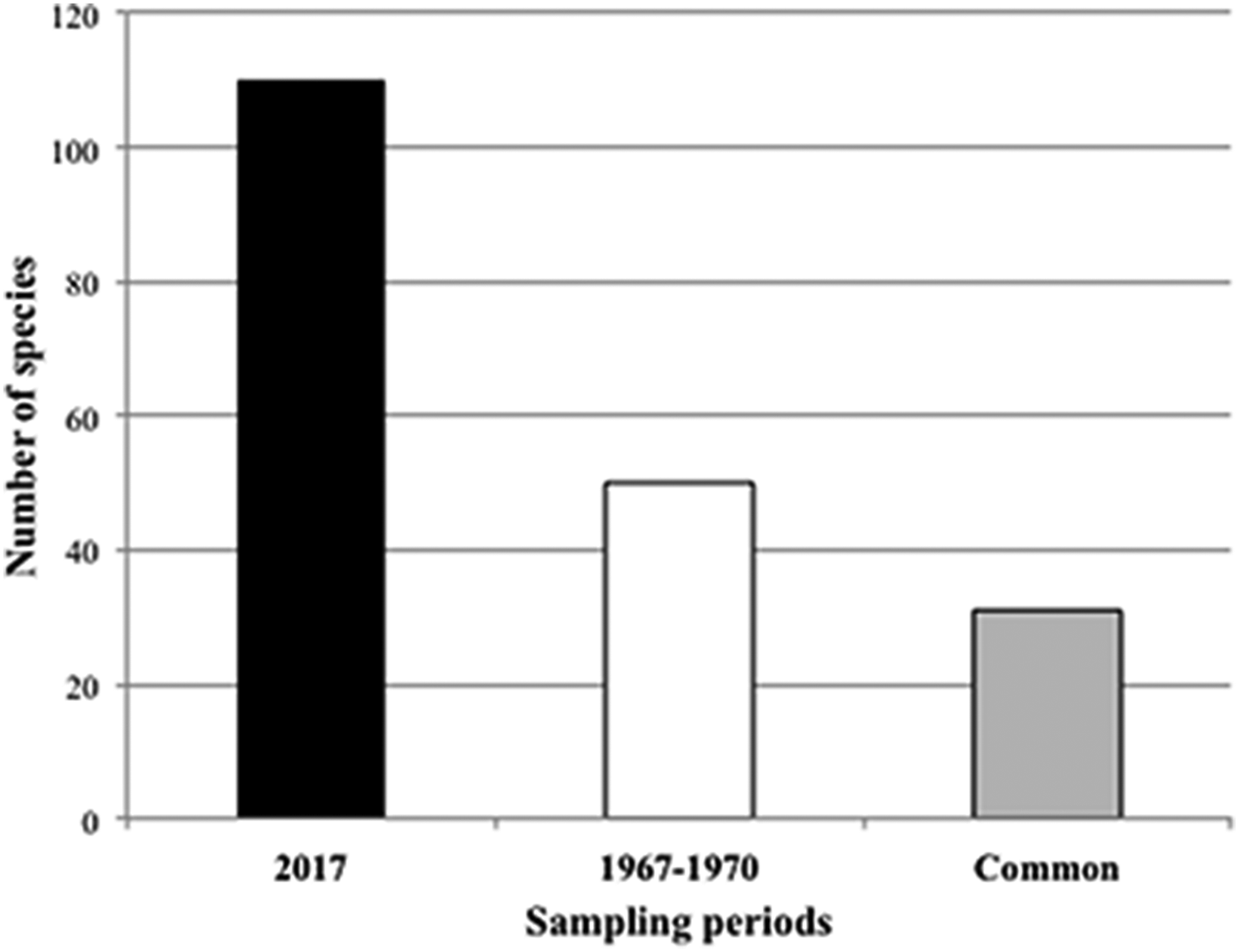
Fig. 7. Number of sponge species recorded during the two sampling periods. The grey bar represents the species in common between the two sampling periods.
The dramatic increase of the species recorded in the recent sampling is probably due to several factors. Firstly, during the recent survey we examined in detail two habitats, marine caves and deep cliffs (finding 13 and five species respectively), that were only sporadically visited by Pulitzer-Finali who found four species in each of them. Moreover, it is very likely that our survey could have been more accurate compared with that of Pulitzer-Finali which collected only discrete and large specimens. On the other hand, if we compare only the species recorded by Pulitzer-Finali in the two most species-rich habitats, the rocky cliffs and the coralligenous, we see that 64.7 and 75% of the species recorded in the period 1967–70 are present currently, indicating a significant resilience of the past communities.
Nevertheless the increase in the number of recorded species should not be considered only in relation to differences in the sampling accuracy. In the two more species-rich habitats, rocky cliffs and coralligenous, the number of species increased from 17 to 26 and from 36 to 77 respectively. This impressive increase was approximately the same in all the considered sponge growth forms (Figure 8).

Fig. 8. Number of sponge species recorded during the two sampling periods divided according to their habit. ME: massive habit, Ec: encrusting habit, CD: cavity dwelling habit, Br: boring habit.
Thibaut et al. (Reference Thibaut, Pinedo, Torras and Ballesteros2005) had hypothesized that, under the recent seawater warming, the Mediterranean benthic communities experienced a strong decrease in biodiversity. Our data indicate that sponge communities do not confirm this assumption. Nevertheless, the strong increase of biodiversity recorded in the present survey may be partially biased by the more accurate sampling method (with coralligenous slices), but very likely indicates a real trend of the community. These data match well with previous evidence from other Mediterranean localities. For example, Costa et al. (Reference Costa, Betti, Nepote, Cattaneo-Vietti, Pansini, Bavestrello and Bertolino2018) have recently shown that the number of sponge species present in semi-submerged caves of the Ligurian Sea surveyed 50 years ago is now almost doubled.
Certainly Porifera, and particularly the horny sponges, suffered many massive epidemic diseases that have brought entire populations of some species to the brink of extinction (Gaino & Pronzato, Reference Gaino and Pronzato1991; Rizzello et al., Reference Rizzello, Corriero, Scalera-Liace and Pronzato1997; Pronzato, Reference Pronzato1999; Cerrano et al., Reference Cerrano, Bavestrello, Bianchi, Cattaneo-Vietti, Bava, Morganti, Morri, Picco, Sara, Schiaparelli, Siccardi and Sponga2000; Pronzato & Manconi, Reference Pronzato and Manconi2008; Pronzato et al., Reference Pronzato, Ledda, Manconi, Lucas and Borja2012; Di Camillo & Cerrano, Reference Di Camillo and Cerrano2015). Nevertheless, evidence coming from comparative studies at pluri-decennial scale, from several areas of the Mediterranean Sea, shows a clear increasing trend of sponge diversity in this period of water warming (Bianchi et al., Reference Bianchi, Morri and Pronzato2014; Bertolino et al., Reference Bertolino, Betti, Bo, Cattaneo-Vietti, Pansini, Romero and Bavestrello2016).
These hypotheses are in agreement with data on a millennial time span, obtained through the analysis of the spicular remains present in dated core samples of coralligenous concretions, indicating an increase of sponge diversity in correspondence with periods of increasing water temperature (Bertolino et al., Reference Bertolino, Cattaneo-Vietti, Costa, Pansini, Fraschetti and Bavestrello2017b).
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0025315419000651.
Acknowledgements
We wish to thank the Avamposto MARE, Università del Salento, for the logistical support.
Financial support
This study was financially supported by SIR grants (Italian Ministry for the Education #RBSI14CH49) and RITMARE project.
Ethical standards
All applicable international, national and/or institutional guidelines for the care and use of animals were followed by the authors.
Sampling and field studies
All necessary permits for sampling and observational field studies have been obtained by the authors from the competent authorities.














