1. Introduction
Dryland forests constitute the largest portion of Ethiopia’s forest resources and are compositionally rich in endemic species (Lemenih & Kassa Reference Lemenih and Kassa2011). These forests are also rich in woody genera like Boswellia, Commiphora and Acacia comprising several indigenous tree species renowned for producing economically valuable oleo-gum resins, including frankincense, myrrh and gum arabica (Alemu et al. Reference Alemu, Pretzsch, El-Sheikh and Omar2012; Tadesse et al. Reference Tadesse, Desalegn and Alia2007; Yogi et al. Reference Yogi, Kumar and Jaiswal2017). The applications of these oleo-gum resins span various industries, including food, pharmaceuticals, perfumery, adhesives, ink and dye (Lemenih & Kassa Reference Lemenih and Kassa2011; Yogi et al. Reference Yogi, Kumar and Jaiswal2017). Moreover, these resins are internationally traded commodities, contributing significantly to the National Gross Domestic Product of several countries, including Ethiopia, Sudan, Somalia and Eritrea (Khamis et al. Reference Khamis, Siddig, Khalil and Csaplovics2016; Lemenih & Kassa Reference Lemenih and Kassa2011).
The genus Boswellia has 24 tree species (Thulin Reference Thulin2020), of which only five produce tradable amounts of oleo-gum resins. Among them, Boswellia papyrifera (Del.) Hochst stands out for its globally tradable aromatic resin known as frankincense (Gebrehiwot et al. Reference Gebrehiwot, Muys, Haile and Mitloehner2003). This product is distinguished by its high levels of octyl acetate and incensole acetate (DeCarlo et al. Reference DeCarlo, Agieb, Johnson, Satyal and Setzer2022). B. papyrifera is found in Ethiopia, Sudan, South Sudan, Eritrea, Uganda and Chad (Gebrehiwot et al. Reference Gebrehiwot, Muys, Haile and Mitloehner2003). Beyond its economic significance, this species provides substantial ecological and cultural values in these regions (Canney-Davison et al. Reference Canney-Davison, Bongers and Phillips2022; Moens et al. Reference Moens, Jacob, Lanckriet, Nyssen, Jacob and Frankl2019). For instance, in 2014, Ethiopia exported approximately 8,000 tons of frankincense valued at 8.8 million US dollars, making the country a major global producer (Tadesse et al. Reference Tadesse, Dejene, Zeleke and Desalegn2020). The collection, processing and grading of frankincense also contribute to the livelihoods of many rural households (Mekonnen et al. Reference Mekonnen, Worku, Yohannes, Bahiru, Mebratu and Teketay2013; Tilahun et al. Reference Tilahun, Muys, Mathijs, Kleinn, Olschewski and Gebrehiwot2011). Furthermore, the species is used for animal fodder, apiculture and soil and water conservation (Gidey et al. Reference Gidey, Hagos, Juhar, Solomon, Negussie, Crous-Duran, Oliveira, Abiyu and Palma2020; Mekonnen et al. Reference Mekonnen, Worku, Yohannes, Bahiru, Mebratu and Teketay2013).
Despite the wider socio-economic and ecological values of B. papyrifera, its population is currently declining at an alarming rate due to over-exploitation, agricultural expansion and habitat degradation (Bongers et al. Reference Bongers, Groenendijk, Bekele, Birhane, Damtew, Decuyper, Eshete, Gezahgne, Girma, Khamis, Lemenih, Mengistu, Ogbazghi, Sass-Klaassen, Tadesse, Teshome, Tolera, Sterck and Zuidema2019; Bongers & Tennigkeit Reference Bongers and Tennigkeit2010; Canney-Davison et al. Reference Canney-Davison, Bongers and Phillips2022; Derero et al. Reference Derero, Worku and Kassa2018; Eshete et al. Reference Eshete, Kassa and Livingstone2021; Ogbazghi et al. Reference Ogbazghi, Rijkers, Wessel and Bongers2006). In addition, the species is now associated with a lack of natural regeneration and little recruitment due to over-exploitation and habitat degradation across its growing areas in Ethiopia, Eritrea and Sudan (Eshete et al. Reference Eshete, Sterck and Bongers2011; Gidey et al. Reference Gidey, Hagos, Juhar, Solomon, Negussie, Crous-Duran, Oliveira, Abiyu and Palma2020; Groenendijk et al. Reference Groenendijk, Eshete, Sterck, Zuidema and Bongers2012; Khamis et al. Reference Khamis, Siddig, Khalil and Csaplovics2016; Ogbazghi Reference Ogbazghi2001). As a result, the remaining natural stands of the species consist mainly of old trees, with few seedlings and saplings distributed across a significant part of its range (Bongers et al. Reference Bongers, Groenendijk, Bekele, Birhane, Damtew, Decuyper, Eshete, Gezahgne, Girma, Khamis, Lemenih, Mengistu, Ogbazghi, Sass-Klaassen, Tadesse, Teshome, Tolera, Sterck and Zuidema2019). While temporary establishment and survival of B. papyrifera seedlings were reported elsewhere (Hizikias Reference Hizikias2011; Negussie et al. Reference Negussie, Aerts, Gebrehiwot and Muys2008; Ogbazghi Reference Ogbazghi2001), their transition into the sapling stage was hindered by several factors, including overgrazing, drought, fire, erosion and insect and pests attacks (Abiyu et al. Reference Abiyu, Bongers, Eshete, Gebrehiwot, Kindu, Lemenih, Moges, Ogbazgh and Sterck2010; Eshete et al. Reference Eshete, Teketay and Hulten2005; Gidey et al. Reference Gidey, Hagos, Juhar, Solomon, Negussie, Crous-Duran, Oliveira, Abiyu and Palma2020; Groenendijk et al. Reference Groenendijk, Eshete, Sterck, Zuidema and Bongers2012; Negussie et al. Reference Negussie, Aerts, Gebrehiwot and Muys2008). Grazing is a major contributing factor to the limited regeneration and recruitment of trees in many tropical areas (Adam & El Tayeb Reference Adam and El Tayeb2008; Giday et al. Reference Giday, Humnessa, Muys, Taheri and Azadi2018; Gidey et al. Reference Gidey, Hagos, Juhar, Solomon, Negussie, Crous-Duran, Oliveira, Abiyu and Palma2020; Liu et al. Reference Liu, Bian, Zhang, Ahmad and Khan2019) and temperate forests (Husheer et al. Reference Husheer, Robertson, Coomes and Frampton2006; Löf et al. Reference Löf, Barrere, Engman, Petersson and Villalobos2021; Long et al. Reference Long, Brose and Horsley2012).
Seedlings of B. papyrifera are likely to establish better in inaccessible areas where pressure from animals is expected to be low (Ogbazghi et al. Reference Ogbazghi, Rijkers, Wessel and Bongers2006). A grazing exclusion strategy has been shown to enhance seed viability, regeneration and seedling development in B. papyrifera woodlands (Alemu et al. Reference Alemu, Pretzsch, El-Sheikh and Omar2012; Eshete et al. Reference Eshete, Teketay, Lemenih and Bongers2012; Tilahun et al. Reference Tilahun, Muys, Mathijs, Kleinn, Olschewski and Gebrehiwot2011). Based on this, we hypothesised that the protection of B. papyrifera wildlings from anthropogenic disturbances (such as grazing and browsing) would improve their survival, seedling growth and biomass under field conditions. Furthermore, we expected that the effect of this strategy would increase with time of fencing. The objectives of this study were to examine: (1) the survival, growth, biomass and plant characteristics of B. papyrifera wildlings in fenced and non-fenced field conditions; and (2) the growth and biomass allocation of B. papyrifera wildlings over time with varying fencing durations.
2. Methods
2.1 Study area
The field experiment was conducted at Jijike site, Abergelle, Tigray region, northern Ethiopia (Fig. 1). The site lies at 13°26'34'' to 13°33'01'' N and 38°48'05'' to 38°53'33'' E. Within the study site, the altitude varies from 1400 to 1650 metres above sea level (Negussie et al. Reference Negussie, Aerts, Gebrehiwot and Muys2008). The monthly average temperature of the study area was estimated at 25.3°C, with an average total annual rainfall of 445 mm mainly raining between mid–June and August (Mengistu et al. Reference Mengistu, Sterck, Anten and Bongers2012). The dominant soil types of the study area are Cambic arenosols, Chromic cambisols and Leptosols (Hizikias Reference Hizikias2011). The vegetation of the study area is characterised as Combretum-Terminalia and Acacia-Commiphora woodland dominated by B. papyrifera, Ipomea spps, Acacia etbaica and Senna singueana (Gidey et al. Reference Gidey, Hagos, Juhar, Solomon, Negussie, Crous-Duran, Oliveira, Abiyu and Palma2020).

Figure 1. Location of the study area.
2.2 Study plots and seedling identification
The field experiment was conducted under natural conditions of the study site to observe the survival, growth and dry biomass of naturally regenerated B. papyrifera seedlings. A reconnaissance survey was conducted to identify the study experimental plots in B. papyrifera dominated woodlands. For homogenisation of the variability of the experimental plots, soil type, slope, aspect, vegetation cover, stoniness and distance from the canopy of trees were thoroughly considered. A total of 36 rectangular study plots, each measuring 1 m × 1.5 m, were established for monitoring the survival and growth of naturally regenerated B. papyrifera seedlings (referred to as wildlings). During the rainy season, 5–10 seedlings of B. papyrifera were randomly selected and marked with permanent tags in each plot, resulting in a total of 305 identified seedlings across all plots. Eighteen plots were enclosed with mesh wire to protect the seedlings from browsing by both domestic and wild animals. The growth and survival of the B. papyrifera seedlings were monitored over five growing seasons and four consecutive years, specifically at 3, 12, 24, 36 and 48 months, using the established permanent plots.
2.3 Data collection
Data related to seedlings’ survival and performance were collected from both fenced and open (non-fenced) plots. Binary seedling survival data (dead = 0, live = 1) were recorded from marked seedlings in these plots. Additionally, seedling height and root collar diameter (RCD) were measured using a graduated metre and a digital calliper, respectively. The number of fully developed leaves and number of apices were also counted for each seedling. In both fenced and open plots, One to two seedlings per plot were uprooted every growing season to measure the leaf area and biomass of the sampled seedlings. These uprooted seedlings were then transported to the Forestry Laboratory, Mekelle University, Ethiopia for further analysis. At the Laboratory, the leaf area was measured using an AM 100 Leaf area metre (ADC Bioscientific Ltd.), and then the seedlings were divided into leaves, stems and roots sections and allowed to oven-dry at a temperature of 65°C until a constant weight was attained (Mokria et al. Reference Mokria, Mekuria, Gebrekirstos, Aynekulu, Belay, Gashaw and Bräuning2018). The leaf, stem and root dry biomass fractions of each seedling were measured using an electronic balance called the laboratory balance PCE (PCE Instruments Ltd.).
2.4 Calculations and statistics
Specific leaf area, leaf size and specific stem density were measured following the methods of Cornelissen et al. (Reference Cornelissen, Lavorel, Garnier, Díaz, Buchmann, Gurvich, Reich, Steege, Morgan, Van Der Heijden, Pausas and Poorter2003). Seedling ratios (e.g., leaf area ratio, leaf weight ratio), absolute growth rates in sizes (e.g., seedling height, root collar diameter), dry weights (e.g., leaf, stem and root) and specific leaf weight were also calculated according to Hunt (Reference Hunt1990). Seedling survival was analyzed using the generalised linear mixed-effect models (GLMM) with logit link function and binomial distribution (glmer function from the R package “lme4”): Logit (seedling survival) ∼ Management + (1| Plot-ID/Site); where management and site are factors.
To explore the statistical mean differences in seedling performance between the fenced and non-fenced plots, non-parametric tests were employed for various seedling attributes. These attributes included RCD, height, leaf number, leaf area, number of apices, stem dry mass, root dry mass and shoot dry mass as the data did not normally distribute. Moreover, to satisfy the assumptions of normal distribution and homogeneity of variances, the leaf dry mass and seedling dry mass data were natural logarithmic (ln)-transformed before conducting statistical analyses. Specifically, the Mann-Whitney U test and T-test were employed to assess mean differences in seedling size and biomass between the fenced and non-fenced plots. For data analysis, both Statistical Package for the Social Sciences software (version 20) and R software were utilised.
3. Results
3.1 Effect of fencing on seedling survival and diebacks
The likelihood of seedling survival was significantly lower in the open areas (Log odds ratio = 0.33, P < 0.05) compared to fenced plots; the odds of survival are reduced by 67% in open plots (Table 1). The statistical analysis employed GLMM, which considered the hierarchical structure of the data – specifically, the nesting of plot factors within site factors. This approach enhances the accuracy of assessing the relationship between management practices and seedling survival.
Table 1. Results of generalised linear mixed-effect models with logit linked function showing the relationships of seedling survival between fenced and open plots in Abergelle, Tigray region, northern Ethiopia
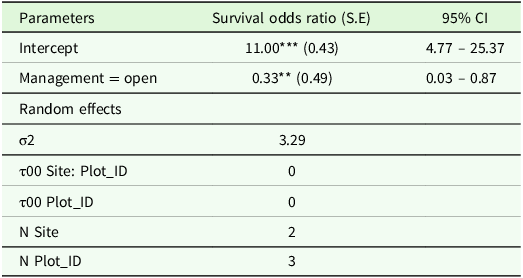
Note: ***Significant at p < 0.001 level and **Significant at p < 0.05 level in the generalized linear mixed-effect models for factors affecting the survival of B. papyrifera wildlings. SE: Stnadard error.
During the wet season (i.e., during the four months of the growing period), B. Papyrifera seedlings exhibited active growth in both above-ground and below-ground traits. However, during the dry period (which spans eight months of growth), B. papyrifera seedlings experienced contrasting responses in their above-ground (shoot) and below-ground (root) growth. While the shoots dried up, the roots remained active and viable as depicted in Fig. 2. This phenomenon likely contributes to the observed higher dry biomass of roots compared to shoots in the fenced and open areas (Fig. 3).

Figure 2. Schematic presentation of dieback behaviour of B. papyrifera seedlings.

Figure 3. Dry biomass of root (black lines) and shoot (blue lines) of B. papyrifera seedlings.
3.2 Effect of fencing on seedling size
Seedlings in the fenced and non-fenced plots differed in almost all seedling growth parameters (Table 2). B. papyrifera seedlings grown in the fenced plots exhibited significantly greater values for RCD, height, leaf numbers, leaf area and number of apices as compared to those in non-fenced plots (Table 2).
Table 2. Mean values of seedling size and biomass of B. papyrifera (Ns = 305) after 4 years under fenced and open experimental plots (Np = 36) in Abergelle, Tigray region, northern Ethiopia
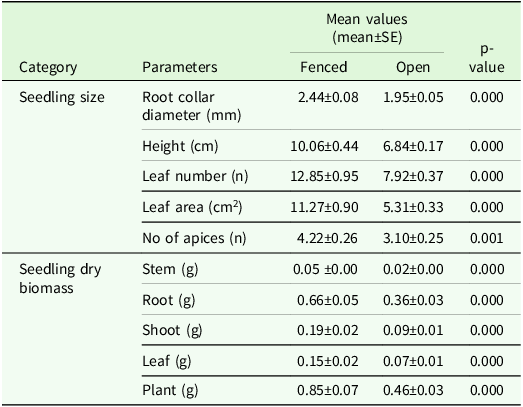
Note: t-test was used to test the mean differences between leaf dry biomass and plant dry biomass, whereas the Mann-Whitney U test was used to test mean differences of all other growth parameters, and tests are significant at P < 0.05.
Dry matter of seedlings grown in the fenced areas was significantly higher in all biomass-related parameters than the seedlings grown in the open environment (Table 2). Seedlings in the fenced plots were 31% higher in total plant dry biomass, 33% in stem biomass, 29% in root biomass, 36% in leaf biomass and 35% in shoot biomass compared to those grown in non-fenced plots conditions. Most of the dry biomass was allocated to roots in both the fenced and non-fenced pots: 77% in the case of fencing and 80% in non-fencing (Table 2).
3.3 Effect of fencing time on seedling size
The size of B. papyrifera seedlings was higher in fenced plots than in the open plots in most fencing times and increased with the fencing time (Fig. 4). Seedling height was significantly different between the fenced and non-fenced plots at 3 and 48 months of fencing times. Besides, the leaf numbers were significantly higher in fenced plots at 36 and 48 months of fencing times. RCD was statistically higher in the fenced plot at 3, 24 and 48 months of fencing times. Seedlings grown in fenced plots also showed significantly higher leaf areas at 3, 36 and 48 months of fencing times (Fig. 4).

Figure 4. Changes in seedling size traits of B. papyrifera seedlings (Ns = 305) with time of fencing. Height (a), RCD (b), leaf numbers (c) and leaf areas (d) in the fenced (black lines) and open (blue lines) plots (Pn = 36). Means significantly different between fenced and open plots are indicated with an asterisk (*) (p < 0.05).
The dry biomass (of most biomass variables) of B. papyrifera seedlings was higher in fenced compared to the non-fenced plots along the growing seasons, and generally, the difference increased with the time of fencing (Fig. 5).

Figure 5. Dry biomass traits of B. papyrifera seedlings (Ns = 305) in the fenced (black lines) and non-fenced (blue lines) plots (Np = 36) during four years of fencing. Leaf (a), stem (b), root (c), shoot (d) and plant (e) dry biomasses. Means significantly different between fenced and open plots are indicated with an asterisk (*) (p < 0.05).
3.4 Effect of fencing on seedling growth rates
Growth rates of B. papyrifera seedlings were higher in the fenced plots compared to the non-fenced plots for most growth variables (Table 3). Specifically, seedling growth rates, including RCD, height, leaf numbers and leaf areas, were approximately 1.04 to 6.6 times greater in the fenced plots than in the non-fenced plots. Likewise, the growth rates in dry biomass variables were nearly 1.1 to 3.8 times higher for the fenced plots compared to the non-fenced plots (Table 3).
Table 3. Absolute growth rates (in size and biomass) of B. papyrifera seedlings under fenced and non-fenced experimental plots in Abergelle, Tigray region, northern Ethiopia

3.5 Seedling functional traits and ratios
The fenced seedlings exhibited greater biomass allocation than the non-fenced counterparts (Table 4). Significantly higher stem weight ratios were recorded in the non-fenced plots compared to the fenced plots. Although the biomass allocation, as reflected by Root:Shoot ratio (R:S), did not show significant differences between the fenced and open plots, the mean R:S ratio of the fenced plots was 10% higher as compared to the non-fenced plots (Table 4).
Table 4. Ratios and traits of seedlings in the fenced and open plots
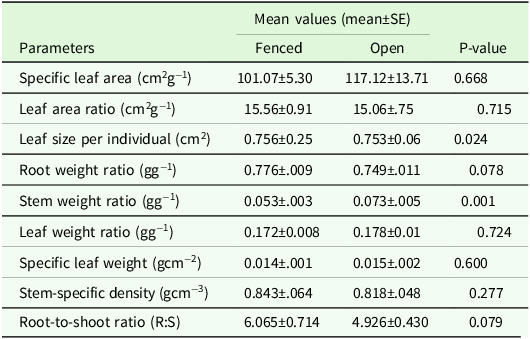
Mann−Whitney U test was used to test mean differences of the parameters, and tests are significant at P < 0.05.
4. Discussion
We conducted an experiment to assess the effect of fencing on the survival and growth of B. papyrifera wildlings. We established permanent experimental plots under field conditions and monitored them for four consecutive years. We hypothesised that the sustained protection of the seedlings from various anthropogenic disturbances (such as grazing and browsing) would improve their survival and growth, ultimately contributing to the natural regeneration of the species in its native habitat. We found that fencing of B. papyrifera wildings did indeed lead to improved seedling survival and performance in terms of size and biomass, leaf area and higher root and leaf biomasses and absolute growth rates.
In agreement with the study results, a positive effect of fencing on B. papyrifera seedling density, recruitment and health conditions was found in exclosures (Moges & Kindu Reference Moges and Kindu2006). Long et al. (Reference Long, Brose and Horsley2012) also reported significantly higher root and stem dry biomasses of red oak (Quercus rubra L.) seedlings within the fenced plots compared to open plots. This enhancement likely facilitated improved water and nutrient uptake, leading to increased growth rates (Bacon 2009). Besides, a higher regeneration, survival rate, RCD and seedling height for several other dryland trees were also found in fenced areas as compared to open systems (Giday et al. Reference Giday, Humnessa, Muys, Taheri and Azadi2018; Long et al. Reference Long, Brose and Horsley2012; Mengistu et al. Reference Mengistu, Teketay, Hulten and Yemshaw2005; Ruo et al. Reference Ruo, Weldegebrial, Yohannes and Yohannes2018; Wassie et al. Reference Wassie, Sterck, Teketay and Bongers2009). For example, Omondi et al. (Reference Omondi, Odee, Ongamo, Kanya and Khasa2017) counted a higher number of seedlings and saplings of Acacia senegal in slightly disturbed populations compared to highly disturbed ones. This could be associated with the lower browsing and trampling of animals on seedlings in the slightly disturbed areas due to their restricted movements (Omondi et al. Reference Omondi, Odee, Ongamo, Kanya and Khasa2017; Wassie et al. Reference Wassie, Sterck, Teketay and Bongers2009). Our study results are also consistent with other studies conducted in dryland regions elsewhere. For instance, Habrova and Pavlis (Reference Habrova and Pavlis2017) observed an increasing rate of seedling survival and development for Dracaena cinnabari with increasing time of their exclosure on the Firmihin Plateau, Socotra Island, Yemen. Similarly, Löf et al. (Reference Löf, Barrere, Engman, Petersson and Villalobos2021) reported a significant height increment in both planted and naturally regenerated Quercues robur seedlings within fenced areas compared to non-fenced areas.
In our study, we observed that the size of the seedlings at the end of the study period (i.e., in the 4th year) remained small. This may be attributed to various factors, such as moisture stress. Plant species in the deciduous dry woodlands have peculiar structural and functional traits that enable them to survive under high disturbance levels such as water deficits and fires (Pulla et al. Reference Pulla, Ramaswami, Mondal, Suresh, Dattaraja, Parthasarathy, Ramesh and Sukumar2015). Under seasonal water deficit conditions, plants either tolerate drought or avoid drought by, for example, dropping leaves to limit transpiration during the dry season. This adaptation allows them to thrive in dry environments. However, in certain regions, for instance, in the African dry woodlands, the intensity and frequency of rainfall can vary considerably even within the short-wet season itself, implying that deciduous trees may face drought stress (Bullock et al. Reference Bullock, Mooney and Medina1995; Murphy & Lugo Reference Murphy, Lugo, Bullock, Mooney and Medina2010). B. papyrifera copes with dry seasons through a conservative strategy using above-ground plant dieback – it dries out during the dry season and experiences re-growth during the wet season (Birhane et al. Reference Birhane, Sterck, Fetene, Bongers and Kuyper2012). Strong variability in rainfall and the occurrence of extended dry spells (water stress) may have significant effects on the annual carbon gain and allocation patterns of B, papyrifera seedlings, affecting their survival in dry areas (Mengistu Reference Mengistu2011). Browsing of the leaves during the wet period reduces the production of photosynthates and thereby the number of reserves in the plant and repeated browsing over the years will reduce the strength of the plant in terms of resprouting capacity (Mengistu Reference Mengistu2011).
Overall, the study findings confirm the beneficial effects of sustained fencing on the survival and growth performance of B. papyrifera seedlings in their natural habitats. Fencing interventions could improve seedling survival and growth, thereby contributing to sustainable conservation of B. papyrifera woodlands. Future research should prioritise investigating the effects of more extended fencing interventions or exclosures (e.g., lasting over 5 years) on B. papyrifera seedlings (and saplings). Furthermore, additional efforts should concentrate on the mechanisms of periodic seedling dieback, and influence of soil moisture and animal browsing thereon.
There is already well established evidence that the sustainability of B. papyrifera woodlands and its renowned product–frankincense, is at greater risk (Bongers et al. Reference Bongers, Groenendijk, Bekele, Birhane, Damtew, Decuyper, Eshete, Gezahgne, Girma, Khamis, Lemenih, Mengistu, Ogbazghi, Sass-Klaassen, Tadesse, Teshome, Tolera, Sterck and Zuidema2019). Several population assessments of B. papyrifera have revealed evidence of the species population and regeneration collapse throughout its natural ranges, necessitating urgent conservation action to manage its regeneration in natural woodlands (Abiyu et al. Reference Abiyu, Bongers, Eshete, Gebrehiwot, Kindu, Lemenih, Moges, Ogbazgh and Sterck2010; Alemu et al. Reference Alemu, Pretzsch, El-Sheikh and Omar2012; Eshete et al. Reference Eshete, Teketay and Hulten2005; Gebrehiwot et al. Reference Gebrehiwot, Muys, Haile and Mitloehner2003; Gidey et al. Reference Gidey, Hagos, Juhar, Solomon, Negussie, Crous-Duran, Oliveira, Abiyu and Palma2020; Groenendijk et al. Reference Groenendijk, Eshete, Sterck, Zuidema and Bongers2012; Ogbazghi et al. Reference Ogbazghi, Rijkers, Wessel and Bongers2006; Tolera et al. Reference Tolera, Sass-Klaassen, Eshete, Bongers and Sterck2013). Several factors contribute to this decline, including overgrazing, reckless tapping for frankincense, fire, land use conversion and insect infestation and damage. These challenges affect the species’ regeneration and seedling performance, and triggered the high adult mortality of the species in Ethiopia (Abiyu et al. Reference Abiyu, Bongers, Eshete, Gebrehiwot, Kindu, Lemenih, Moges, Ogbazgh and Sterck2010; Eshete et al. Reference Eshete, Teketay and Hulten2005; Negussie et al. 2008, Reference Negussie, Gebrehiwot, Yohannes, Norgrove and Aynekulu2021), Eritrea (Ogbazghi et al. Reference Ogbazghi, Rijkers, Wessel and Bongers2006) and Sudan (Khamis et al. Reference Khamis, Siddig, Khalil and Csaplovics2016). B. papyrifera seedlings are sensitive to trampling, browsing and fire, among others. To address this, management options that promote adequate regeneration and long-term sustainability of populations are crucial. Regulated frankincense harvest and intensive population management strategies are essential for the natural stands. The future of this flagship species remains extremely unstable, emphasizing the need for coordinated efforts from all stakeholders to enhance its natural regeneration and preserve its myriad socio-economic and ecological roles (Bongers et al. Reference Bongers, Groenendijk, Bekele, Birhane, Damtew, Decuyper, Eshete, Gezahgne, Girma, Khamis, Lemenih, Mengistu, Ogbazghi, Sass-Klaassen, Tadesse, Teshome, Tolera, Sterck and Zuidema2019; Gidey et al. Reference Gidey, Hagos, Juhar, Solomon, Negussie, Crous-Duran, Oliveira, Abiyu and Palma2020; Lemenih et al. Reference Lemenih, Arts, Wiersum and Bongers2014; Lemenih & Kassa Reference Lemenih and Kassa2011). Based on the results of this study, we suggest the following measures: (i) sustained protection: implement fencing interventions to safeguard the remnant B. papyrifera woodlands, and (ii) restoration: actively restore degraded areas in integration with protection efforts.
In this context, there is well established evidence that converting communal grazing lands into exclosures, primarily using physical fences, offers several conservation benefits. These benefits include vegetation restoration, improvement in soil nutrient status and erosion reduction (Mekuria et al. Reference Mekuria, Veldkamp, Haile, Nyssen, Muys and Gebrehiwot2007; Shimelse et al. Reference Shimelse, Bekele, Nemomissa and Leal Filho2020; Welemariam et al. Reference Welemariam, Kebede, Bedadi and Birhane2018). Besides, the positive roles of exclosures are widely recognised and supported by local communities in dryland areas in Ethiopia, particularly in the Tigray region, northern Ethiopia (Birhane et al. Reference Birhane, Mengistu, Seyoum, Hagazi, Putzel, Rannestad and Kassa2017; Gebregziabher and Soltani Reference Gebregziabher and Soltani2019; Mekonen et al. Reference Mekonen, Hailu and Abitew2022). However, the sustainability and success rate of the intervention are challenged by several factors, including biophysical and institutional factors (Birhane et al. Reference Birhane, Mengistu, Seyoum, Hagazi, Putzel, Rannestad and Kassa2017). Although fenced/exclosure areas primarily benefit local communities through biodiversity enhancement, soil and water condition improvements, and other ecological aspects, the livelihood improvements often receive less attention. Therefore, for the intervention to be both feasible and sustainable, it is crucial to consider the socio-economic and cultural context of the local communities. In light of this, exclosures established using social fences are widely regarded as more favourable in terms of ecology and social acceptance (Birhane et al. Reference Birhane, Mengistu, Seyoum, Hagazi, Putzel, Rannestad and Kassa2017). Ensuring active community participation from the selection of intervention areas to the establishment and management of exclosures is vital. Simultaneously, implementing benefit-sharing mechanisms and strategies among the custodians will contribute to the long-term success of these conservation efforts.
In the same manner, ensuring the sustainable and effective restoration of B. papyrifera woodlands through this intervention necessitates a transition from physical fencing to social fencing in the long term. The feasibility of the intervention for this particular tree species is supported by the fact that the tree species is highly valued by the local community for its versatile roles and that it is highly threatened and in immediate need of conservation actions. In addition, the remaining populations of this tree species are found in mostly inaccessible areas, areas not directly needed for agricultural practices, providing an ideal setting for establishing exclosures to safeguard this flagship species. Although armed conflict currently exerts another strong pressure on the Boswellia forests (Johnson & Bongers Reference Johnson and Bongers2024), the success of this intervention hinges on full community participation at all stages. By engaging local stakeholders, we can ensure the long-term success of this vital conservation endeavour.
Acknowledgements
The authors gratefully acknowledge Wageningen University and Research, and the NORAD II (Ecological Rehabilitation Project) for their financial support to the study. We also acknowledged the Rufford Foundation (grant numbers: 21680-1, 26273-2, 31671-B and 40760-D) and People’s Trust for Endangered Species for their financial support to Tesfay Gidey to participate in the study. Moreover, we are also grateful to the local guards for their valuable support and protection of the study experimental plots.
Financial support
This research was financially supported by PhD sandwich grant from Wageningen University and Research, and NORAD II (Ecological Rehabilitation Project).
Competing of interests
The authors declared that they have no competing of interests exist.
Ethical statement
None.
Publishing ethics
The data and manuscript are our own original work.












