Introduction
The Amazon basin house the highest freshwater fish diversity in the world with at least 2,716 species currently documented for the region (Dagosta and de Pinna, Reference Dagosta and de Pinna2019). Explanations for this high species diversity involved climatic variability, habitat heterogeneity and historical events (Junk et al., Reference Junk, Soares and Bayley2007; Albert et al., Reference Albert, Carvalho, Petry, Holder, Maxime, Espino, Corahua, Quispe, Rengifo, Ortega and Reis2011; Crampton, Reference Crampton, Albert and Reis2011; Dagosta and de Pinna, Reference Dagosta and de Pinna2017; Oberdorff et al., Reference Oberdorff, Dias, Jézéquel, Albert, Arantes, Bigorne, Carvajal-Valleros, Wever, Frederico, Hidalgo, Hugueny, Leprieur, Maldonado, Maldonado-Ocampo, Martens, Ortega, Sarmiento, Tedesco, Torrente-Vilara, Winemiller and Zuanon2019). Most ecological and historical explanations of Amazonian fish diversity are ultimately linked to basin size which affects habitat diversity and promotes more opportunities to speciation (Levêque et al., Reference Levêque, Oberdorff, Paugy, Stiassny and Tedesco2008; Oberdorff et al., Reference Oberdorff, Tedesco, Hugueny, Leprieur, Beauchard, Brosse and Durr2011; Oberdorff et al., Reference Oberdorff, Dias, Jézéquel, Albert, Arantes, Bigorne, Carvajal-Valleros, Wever, Frederico, Hidalgo, Hugueny, Leprieur, Maldonado, Maldonado-Ocampo, Martens, Ortega, Sarmiento, Tedesco, Torrente-Vilara, Winemiller and Zuanon2019). Indeed, with more than 6.8 million km2, the Amazon is the largest river basin in the world and is composed of many large tributaries interconnected with a huge number of smaller rivers (Goulding et al., Reference Goulding, Barthem and Ferreira2003; Venticinque et al., Reference Venticinque, Forsberg, Barthem, Petry, Hess, Mercado, Cañas, Montoya, Durigan and Goulding2016).
Variation in water colour is another remarkable characteristic of Amazonian rivers, which have been categorized into rivers with white-, black- and clearwaters by local peoples and naturalists (Wallace Reference Wallace1889; Sioli, Reference Sioli and Sioli1984; Goulding et al., Reference Goulding, Barthem and Ferreira2003; Venticinque et al., Reference Venticinque, Forsberg, Barthem, Petry, Hess, Mercado, Cañas, Montoya, Durigan and Goulding2016). Water colours of Amazonian rivers reflect their limnological properties such as oxygen contents, pH and turbidity (Silva et al., Reference Silva, Miranda, Domingos, Silva and Santana2013; Ríos-Villamizar et al., Reference Ríos-Villamizar, Piedade, Da Costa, Adeney and Junk2014). In addition, water colours indicate the geomorphological origins of sub-basins since the Amazonian major rivers originated from three main sources: Andes with white colour rivers, Guiana Shield with blackwater rivers and Brazilian Shield with clearwaters rivers (Sioli, Reference Sioli and Sioli1984; Goulding et al., Reference Goulding, Barthem and Ferreira2003).
In this context, river water colours could be useful surrogates for current abiotic conditions as well as the biogeographic context were the Amazonian aquatic biota living and evolved (Beheregaray et al., Reference Beheregaray, Cooke, Chao and Landguth2015). Indeed, there is a growing body of evidence that water colours of Amazonian rivers provide a set of evolutionary and ecological filters for fish geographic distribution and diversification (Pires et al., Reference Pires, Borghezan, Machado, Powell, Röpke, Oliveira, Zuanon and Farias2018; Bogotá-Gregory et al., Reference Bogotá-Gregory, Lima, Correa, Silva-Oliveira, Jenkins, Ribeiro, Lovejoy, Reis and Crampton2020). Surprisingly, the influence of river water colours on Amazonian fish assemblages has been poorly investigated, especially at the larger scales (but see Oberdorff et al., Reference Oberdorff, Dias, Jézéquel, Albert, Arantes, Bigorne, Carvajal-Valleros, Wever, Frederico, Hidalgo, Hugueny, Leprieur, Maldonado, Maldonado-Ocampo, Martens, Ortega, Sarmiento, Tedesco, Torrente-Vilara, Winemiller and Zuanon2019).
In pioneering studies, Henderson and Crampton (Reference Henderson and Crampton1997) and Saint-Paul et al. (Reference Saint-Paul, Zuanon, Correa, Garcia, Fabré, Berger and Junk2000) documenting marked differences in fish assemblages found in black- and whitewater lakes and rivers. More recently, Bogotá-Gregory et al. (Reference Bogotá-Gregory, Lima, Correa, Silva-Oliveira, Jenkins, Ribeiro, Lovejoy, Reis and Crampton2020) found higher species richness, biomass and distinctive assemblage composition in nutrient-rich whitewater lakes compared to oligotrophic black- and clearwater rivers. Those studies suggest that fish species turnover between Amazonian rivers could be, at least partially, predicted by their water colours.
The investigation of the influence of water types on Amazonian fish assemblages is limited by data on fish species distribution and rivers’ limnological properties. Fortunately, efforts to synthesize data (Silva, Reference Silva2013; Silva et al., Reference Silva, Miranda, Domingos, Silva and Santana2013, Dagosta and de Pinna, Reference Dagosta and de Pinna2017, Reference Dagosta and de Pinna2019; Jézéquel et al., Reference Jézéquel, Tedesco, Bigorne, Maldonado-Ocampo, Ortega, Hidalgo, Martens, Torrente-Vilara, Zuanon, Acosta, Agudelo, Barrera Maure, Bastos, Bogotá-Gregory, Cabeceira, Canto, Carvajal-Vallejos, Carvalho, Cella-Ribeiro, Covain, Donascimiento, Doria, Duarte, Ferreira, Galuch, Giarrizzo, Leitão, Lundberg, Maldonado, Mojica, Montag, Ohara, Pires, Pouilly, Prada-Pedreros, de Queiroz, Rapp Py-Daniel, Ribeiro, Ríos Herrera, Sarmiento, Sousa, Stegmann, Valdiviezo-Rivera, Villa, Yunoki and Oberdorff2020) permit to examine the relationship between Amazonian rivers’ types and the turnover of fish species at larger scales. We present here a literature-based study aimed to investigate the relationships between fish species composition and water colour for some major Amazonian rivers. In addition to local scale studies mentioned before, we attempt to connect the fish species turnover with limnological properties at sub-basin scale.
We expect that physical and chemical properties of distinct river types will affect the turnover of fish species composition which, in turn, could be predicted by their respective colours. Distance between rivers could be also relevant to explain dissimilarity in species composition at biogeographic scale (Nekola and White, Reference Nekola and White1999). Following these considerations, the main questions guiding our study were as follows: (a) Are there differences in the species richness of major fish lineages between rivers with distinct water colours? (b) What is the degree of species dissimilarity between rivers with different water colours? (c) Do variations in the physical and chemical properties of contrasting river waters are correlated with fish species turnover? and (d) Can fish species dissimilarity also be explained by distance between major rivers?
Methods
Study area
We chose eight major Amazonian rivers conditioned to have data on fish species composition and water physicochemical properties in the consulted literature (Figure 1): two with whitewater (Purus and Juruá), three blackwaters (Negro, Urubu and Uatumã) and three with clearwaters (Tapajós, Juruena and Xingu). Data on Urubu and Uatumã rivers is combined since fish species distribution are not separated in the consulted database (see below). We also merged data from Tapajós and its tributary Juruena since no abiotic data were available from the latter river in the consulted literature (Silva, Reference Silva2013).

Figure 1. Map of Amazon basin showing in brown lines the rivers considered in this study. The yellow circles are the middle rivers’ position whose geographic coordinates were used to calculate distance between rivers (see Methods section).
The whitewater rivers are located in southwestern Amazon region, and their valleys cover 217,000 km2 (Juruá) and 375,000 km2 (Purus) with headwaters flowing from Peruvian Amazon (Goulding et al., Reference Goulding, Barthem and Ferreira2003). Both rivers are highly meandered and sediment-rich, contributing together with 7% of annual discharge to the Amazon (Goulding et al., Reference Goulding, Barthem and Ferreira2003). The Xingu and Tapajós/Juruena rivers are located in eastern Brazilian Amazon draining through the Brazilian shield. These low-input sediment rivers are characterized by cataracts and rapids along its courses (Goulding et al., Reference Goulding, Barthem and Ferreira2003). Negro and Urubu rivers drained through the ancient Guiana Shield being recognized by its typical dark waters. Negro valley covers 700,000 km2, being the second largest Amazon River tributary (Goulding et al., Reference Goulding, Barthem and Ferreira2003).
Data collection
Fish records were obtained from a recent database compiled and provided as supplementary material in Dagosta and de Pinna (Reference Dagosta and de Pinna2017) that directly examined specimens in the main ichthyologic collections of Amazonian fishes and made an exhaustive and careful literature review. Dagosta and de Pinna (Reference Dagosta and de Pinna2017) divided the Amazon basin in 29 regions that partially coincide with major sub-basin. We merged data of some of those regions (e.g. lower and upper Xingu River) to have a checklist at sub-basin scale.
Fishes records in Dagosta and de Pinna (Reference Dagosta and de Pinna2017) were obtained from primary sources (institutional collections) and more than 1,500 literature sources covering decades of fish sampling in Amazonian rivers. Unfortunately, due heterogeneity of consulted sources, detailed information such as fish abundance, sampling effort, methods applied, habitats and locations sampled are not specified by each river. Fish records available in Dagosta and de Pinna (Reference Dagosta and de Pinna2017), for instance, do not permit to assign each species to specific locations at main river channel or its tributaries. Data from Purus and Juruá rivers also include some fish records from neighbourhood rivers (e.g. Jataí). Even with these limitations, this is likely the most complete available database at large scale of Amazonian fish distribution compiled to date (Dagosta and de Pina, Reference Dagosta and de Pinna2017, Reference Dagosta and de Pinna2019, see also Jézéquel et al., Reference Jézéquel, Tedesco, Bigorne, Maldonado-Ocampo, Ortega, Hidalgo, Martens, Torrente-Vilara, Zuanon, Acosta, Agudelo, Barrera Maure, Bastos, Bogotá-Gregory, Cabeceira, Canto, Carvajal-Vallejos, Carvalho, Cella-Ribeiro, Covain, Donascimiento, Doria, Duarte, Ferreira, Galuch, Giarrizzo, Leitão, Lundberg, Maldonado, Mojica, Montag, Ohara, Pires, Pouilly, Prada-Pedreros, de Queiroz, Rapp Py-Daniel, Ribeiro, Ríos Herrera, Sarmiento, Sousa, Stegmann, Valdiviezo-Rivera, Villa, Yunoki and Oberdorff2020).
We also compiled information on the physical and chemical properties of the waters of each river using the data available in Silva (Reference Silva2013) and Silva et al. (Reference Silva, Miranda, Domingos, Silva and Santana2013). The following continuous abiotic parameters were used in the analysis: pH, colour (mg Pt/L), turbidity (NTU), dissolved oxygen (DO – mg/L), ammonia (NH4 – mg/L) and total suspended solids (TSS – mg/L). These data were collected from 2009 to 2012 in 189 sampling sites distributed along the Amazon river tributaries (e.g. Purus, Negro) using cities located along major rivers as geographic reference (Silva, Reference Silva2013).
Physical and chemical parameters of river waters were obtained during low and high water periods (Silva, Reference Silva2013). Some of these parameters present seasonal variation along the hydrological cycles (Silva, Reference Silva2013). Therefore, to control for seasonality, in our analysis we used average values of each parameter across low and high water seasons. Details on field and laboratory procedures and sampling site distribution are found in Silva (Reference Silva2013) and Silva et al. (Reference Silva, Miranda, Domingos, Silva and Santana2013).
Data analysis
Our study is based on independent investigations with distinct aims, sampling effort and design which are major drawbacks to analyse fish assemblage-environment relationships. To ameliorate some of these limitations, our analysis focus in proportional diversity and compositional metrics little affected by differences in species richness. We use proportional Z tests (Zar, Reference Zar2010) to compare species richness within major fish lineages (Orders) in paired river types. Taxonomic rank at Order resolution was based on Sleen and Albert (Reference Sleen and Albert2018). Critical P values were adjusted to 0.02 using Bonferroni criterion since we made multiple comparisons between river types.
Distributional data were synthetized in a presence or absence matrix of each species in each river basin. Variations in fish species composition between rivers were examined with hierarchical cluster analysis with group averaging as the linkage method and βsim as the dissimilarity measurement. βsim is a measure of species turnover weakly affected by differences in species richness (Baselga, Reference Baselga2012; Leprieur and Oikonomou, Reference Leprieur and Oikonomou2017) which allows comparing samples collected with different sampling efforts. Values for βsim range from 0 (low dissimilarity, identical species list) to 1 (high dissimilarity, no shared taxa).
Between-river dissimilarity in their abiotic properties was also examined with hierarchical cluster analysis using Euclidian distances. The values of abiotic parameters were transformed (Log x +1) and normalized, since the physicochemical data were highly variable and measured in different units.
We examine the correlation between river’s abiotic characteristics and dissimilarity in fish assemblages using the Relate procedure. Relate is a non-parametric type of Mantel test used to test the hypothesis of ‘no agreement in multivariate pattern’ of two independently derived distance matrices (Clarke and Warwick, Reference Clarke and Warwick2001). The agreement between matrices is measured through a Spearman (Rho) rank correlation coefficient (Clarke and Warwick, Reference Clarke and Warwick2001). In this analysis, we compared two matrices: one based on βsim values (biotic data) and another using Euclidian distances (abiotic data). If physical and chemical properties of river waters affect fish species composition, we expected to find a high and significant correlation between those matrices.
The Relate procedure was also applied to examine the influence of distance among rivers in the fish species composition. Since the Dagosta and de Pinna (Reference Dagosta and de Pinna2017) did not include detailed geographic information about rivers, we took geographic coordinates in an arbitrary point in the middle course of the rivers (Figure 1). We used these coordinates to calculate distances between rivers. We use the coordinates of the junction of Tapajós and Juruena rivers, and in Uatumã/Urubu, we considered only the Uatumã coordinates in the analysis (Figure 1). We prepared a matrix of geographical distance among rivers, and the correlation between the geographic distance and fish dissimilarity matrices was also tested with Relate procedure described above. Relate and cluster analyses were performed in Primer 6 (Clarke and Warwick, Reference Clarke and Warwick2001).
Results
The data provided by Dagosta and de Pinna (Reference Dagosta and de Pinna2017) contained a total of 1,219 fish species in the study rivers which represents nearly 45% of documented Amazonian fish diversity (Dagosta and de Pinna Reference Dagosta and de Pinna2019), distributed as follows: Negro River (644 species), Xingu River (465), Purus River (410), Tapajós/Juruena rivers (373), Juruá River (286) and Urubu/Uatumã rivers (203 species). The black- and clearwater rivers sampled shared 29% of their fish species, the same proportion shared by black- and whitewaters rivers. A lower proportion (23%) of fish species was shared by clear- and whitewater rivers. The three river types housed 175 fish species in common (14%), and only 23 species (2% of total) were recorded in all rivers.
The four major fish lineages show significant differences in their contribution to diversity of river types. Characiformes were proportionally more diverse in clearwaters compared to whitewater rivers, especially in the Tapajós/Juruena (Table 1). Siluriformes and Gymnotiformes were proportionally more diverse in whitewater rivers (Table 1). In contrast, Perciformes tended to be better represented in black- and clearwaters compared to whitewater rivers (Table 1).
Table 1. Number of species and percentage of ichthyofauna represented by major fish lineages in selected Amazonian rivers. River types with different caps letters had statistical differences in proportional species richness. Data from Dagosta and Pinna (Reference Dagosta and de Pinna2017). In paired comparisons, different uppercase letters means statistical differences in species richness proportions (P < 0.001)

* Marginally significant P = 0.02.
The βsim values were usually above 0.50 in comparisons of rivers with distinct colours and indicate that species turnover is highest between such pairs. Indeed, based on fish species composition, rivers were grouped according to their water colours (Figure 2). Black- and clearwater rivers tend to be more similar to each other as mentioned above. In contrast, whitewater rivers had a very dissimilar fish composition compared to other river types (Figure 2).
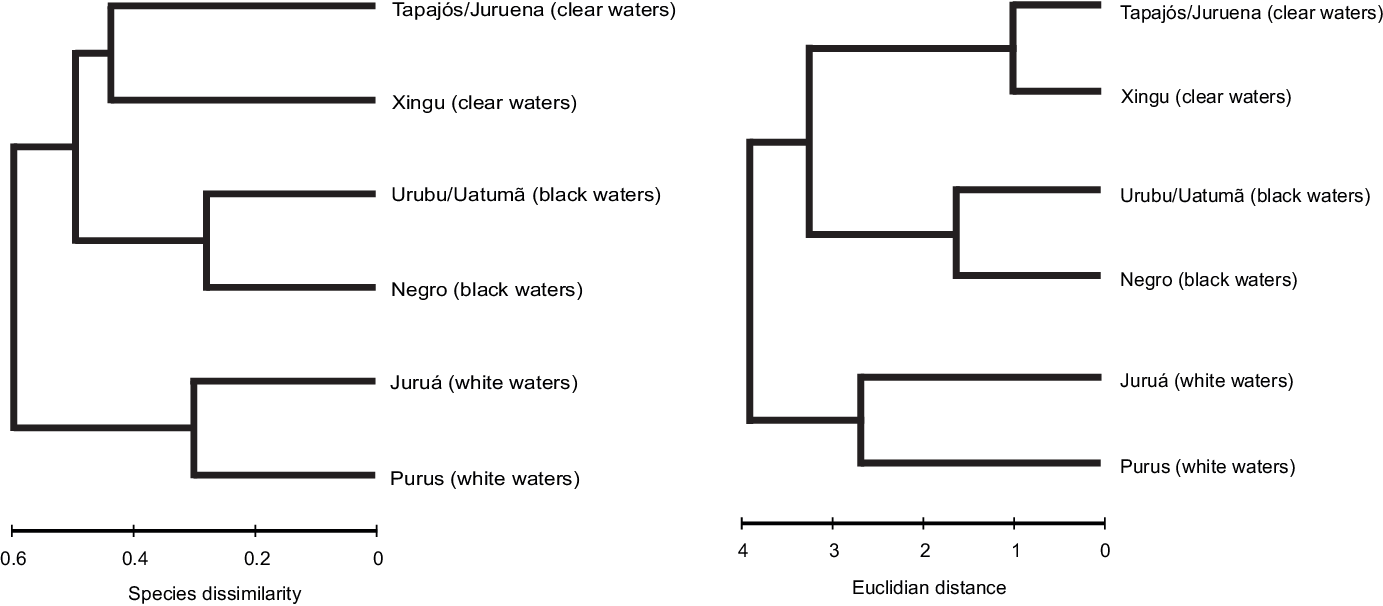
Figure 2. Grouping Amazonian rivers after their fish species dissimilarity (left graph) and abiotic environmental distance (right graph).
Based on the data provided by Silva (Reference Silva2013) and Silva et al. (Reference Silva, Miranda, Domingos, Silva and Santana2013), the rivers selected present great contrasts in physical and chemical properties of their waters. Clearwater rivers (Xingu and Tapajós/Juruena) had the lowest values in colour measurements, turbidity, ammonia and quantity of suspended materials, but high contents of dissolved oxygen (Table 2). Higher values of turbidity and suspended materials were found in Purus and Juruá rivers (Table 2). Acidic, strongly coloured water with high ammonia contents characterized the blackwater rivers (Table 2). Rivers were grouped by shared abiotic properties in the same way as did for the fish species composition (Figure 2).
Table 2. Physical and chemical properties of rivers considered in this study. Data from Silva (Reference Silva2013) and Silva et al. (Reference Silva, Miranda, Domingos, Silva and Santana2013)
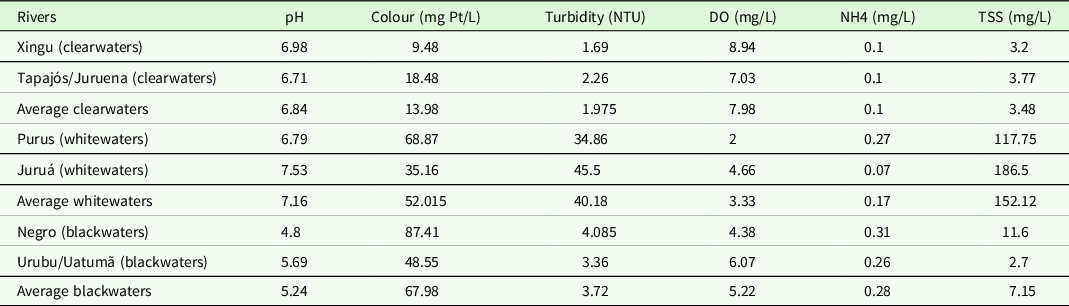
Legends: DO (dissolved oxygen), NH4 (ammonia), TSS (total suspended solids).
Matrices based on species dissimilarity and abiotic data were significantly associated with each other (Relate test, 999 permutation, Rho = 0.68, P = 0.01), indicating that increasing differences in water physical and chemical parameters implied in increasing dissimilarity in fish species composition (Figure 3). Fish species composition is also related to distance among rivers (Relate test, 999 permutation, Rho = 0.69, P = 0.003) with high species dissimilarity observed in rivers far from each other (Figure 3).

Figure 3. Relationship between dissimilarity in fish species composition and environmental distance (left graph) and geographic distance (right graph) in major Amazonian rivers. Each point represents a pair of rivers whose abbreviations are: X (Xingu – clearwaters), T (Tapajós/Juruena – clearwaters), N (Negro – blackwaters), P (Purus – whitewaters), J (Juruá – whitewaters), and U (Urubu/Uatumã – blackwaters). Note that rivers with contrasting water colour had higher environmental distance and higher fish species dissimilarity.
Discussion
Identifying the variables affecting fish assemblages in the highly heterogeneous and dynamic aquatic ecosystems of Amazonia is a major challenge (Oberdorff et al., Reference Oberdorff, Dias, Jézéquel, Albert, Arantes, Bigorne, Carvajal-Valleros, Wever, Frederico, Hidalgo, Hugueny, Leprieur, Maldonado, Maldonado-Ocampo, Martens, Ortega, Sarmiento, Tedesco, Torrente-Vilara, Winemiller and Zuanon2019), and our proposal was not to explore all the potential variables (e.g. climate, vegetation cover) that affects fish distribution. Our aim with this modest contribution was to evaluate the water colour as a simple predictive variable to fish species turnover at large scale by taking advantage of accessible literature. However, the literature-based approach used here had important limitations, especially the lack of temporal and spatial overlap in the sampling fishes and rivers’ abiotic proprieties. Also, the absence of quantitative data is a major disadvantage to analysis at individual fish species. Nevertheless, we believed that our study had heuristic value to understand the relevance of river water colour to fish species turnover at large scale.
According to our expectations, Amazonian rivers with contrasting water colours possess different abiotic attributes (see Silva et al., Reference Silva, Miranda, Domingos, Silva and Santana2013 for a larger sample of rivers) and also distinct fish species composition. However, dissimilarity in fish species composition could also be explained by distance between rivers and disentangling the effects of environmental and spatial distance on the species composition it is not possible due our limited sample of rivers. We argue, however, that geographic and environmental distances could represent ultimate (river basins histories – Hubert and Renno, Reference Hubert and Renno2006; Dagosta and de Pinna, Reference Dagosta and de Pinna2017) and proximate causes (physiology and habitat use – Val and Almeida-Val, Reference Val and Almeida-Val1995; Crampton, Reference Crampton, Albert and Reis2011) of fish species distribution.
River basins near each other likely shared a common history in their development and also had similar fish faunas. Indeed, the black- and clearwaters rivers considered in our study runs through the highly eroded and ancient Guiana and Brazilian shield, respectively, while the whitewater rivers drained through sedimentary basins affected by Andean orogeny (Sioli Reference Sioli and Sioli1984; Goulding et al., Reference Goulding, Barthem and Ferreira2003). The fish species turnover documented in our study could be ultimately driven by the geological history of rivers which provides different opportunities to speciation and biotic interchange. Indeed, some authors suggest hydrological rearrangements associated with Andean foreland dynamics (Hubert and Renno, Reference Hubert and Renno2006), and drainage capture (Dagosta and de Pinna, Reference Dagosta and de Pinna2017) had strong effects on Amazonian fish distribution.
Regarding the proximate causes of fish distribution, our results suggest that water colours can be useful proxies for abiotic filters that affect species with distinct physiological and ecological requirements (Bogotá-Gregory et al., Reference Bogotá-Gregory, Lima, Correa, Silva-Oliveira, Jenkins, Ribeiro, Lovejoy, Reis and Crampton2020). We consider that proximate abiotic filters affect fish species distribution directly and indirectly. Direct effects involve the action of physical and chemical properties of water as physiological filters to fish distribution (Val & Almeida-Val Reference Val and Almeida-Val1995; Barletta et al., Reference Barletta, Jaureguizar, Baigun, Fontoura, Agostinho, Almeida-Val, Val, Torres, Jimenes-Segura, Giarrizzo, Fabré, Batista, Lasso, Taphorn, Costa, Chaves, Vieira and Corrêa2010). Some of the water parameters such as pH and oxygen dissolved have so extreme values that they are likely to select species via their limits of physiological tolerance. Indeed, both laboratory and field data demonstrate that different water types impose a wide variety of environmental challenges to Amazonian fishes’ physiological adaptation, and affect species distributions at the regional scale (Val and Almeida-Val, Reference Val and Almeida-Val1995; Barletta et al., Reference Barletta, Jaureguizar, Baigun, Fontoura, Agostinho, Almeida-Val, Val, Torres, Jimenes-Segura, Giarrizzo, Fabré, Batista, Lasso, Taphorn, Costa, Chaves, Vieira and Corrêa2010; Duncan and Fernandes, Reference Duncan and Fernandes2010). In such circumstances, the interaction between abiotic attributes of waters and the fishes’ physiological tolerance can contribute to species turnover.
Indirect effects of abiotic filters imply habitat specialization by fish species. The Amazon fish fauna is composed of species with diversified natural history strategies, including selection of distinct habitats such as lakes, flooded forest, floating meadows, litter banks, waterfalls among others (Henderson & Crampton Reference Henderson and Crampton1997; Saint-Paul et al. Reference Saint-Paul, Zuanon, Correa, Garcia, Fabré, Berger and Junk2000; Carvalho et al., Reference Carvalho, Zuanon, Sazima and Del-Claro2007; Barletta et al., Reference Barletta, Jaureguizar, Baigun, Fontoura, Agostinho, Almeida-Val, Val, Torres, Jimenes-Segura, Giarrizzo, Fabré, Batista, Lasso, Taphorn, Costa, Chaves, Vieira and Corrêa2010; Crampton Reference Crampton, Albert and Reis2011; Carvalho et al., Reference Carvalho, Fidelis, Arruda, Galuch and Zuanon2013). The occurrence of some habitats and microhabitats used by fishes apparently is also related to rivers with distinct colours. For example, rocky outcrops and waterfalls are more common in black- and clearwater rivers, while floating meadows are typical of whitewater rivers (Goulding et al., Reference Goulding, Barthem and Ferreira2003; Piedade et al., Reference Piedade, Junk, D’Ângelo, Wittmann, Schöngart, Barbosa and Lopes2010). Thus, the distribution of distinct habitats selected by specialized fish species could partially explain the species turnover associated with rivers with contrasting colours.
Differences in species diversity of some fish lineages between river types could be also associated with environmental filters acting as proximate causes of fish distribution mentioned earlier. For example, the Gymnotiformes is a fish lineage tolerant to anoxic conditions and particularly associated with floating meadows in whitewater floodplain lakes in western Amazon (Henderson and Crampton, Reference Henderson and Crampton1997). Siluriformes and Gymnotiformes were also abundant in whitewater river in a region intensively sampled by Bogotá-Gregory et al. (Reference Bogotá-Gregory, Lima, Correa, Silva-Oliveira, Jenkins, Ribeiro, Lovejoy, Reis and Crampton2020) in accordance with our analysis. These results indicate that diversity of some major Amazonian fish lineages is associated with rivers with distinct water colours.
As mentioned before, our literature-based approach had limitations, and to verify the generality of the patterns documented here, it will be necessary to increase the number of rivers simultaneous sampled for fish assemblages and abiotic data. It is important to note that, even within major Amazonian river basins, it is possible to find tributaries with contrasting water colours such as Branco River, a whitewater tributary of the Negro River (blackwater). Sampling fishes in such ecological contexts could be an interesting approach to test the relevance of water colours for fish species turnover at sub-regional scale with a better control of other effects such as spatial distance (Bogotá-Gregory et al., Reference Bogotá-Gregory, Lima, Correa, Silva-Oliveira, Jenkins, Ribeiro, Lovejoy, Reis and Crampton2020, see also Laranjeiras et al., Reference Laranjeira, Naka and Cohn-Haft2019).
Species functional diversity is also affected by the proximate causes of Amazonian fish distribution (Benone et al., Reference Benone, Leal, dos Santos, Mendes, Heino and de Assis Montag2020) and a promising line of investigation is to determine which fish’s ecological traits are filtered by rivers with distinct colours to understand functional responses of fish assemblage to water abiotic properties. For instance, the sensorial and communication traits of Siluriformes and Gymnotiformes apparently are filtered by water with contrasting physicochemical properties (Henderson and Crampton Reference Henderson and Crampton1997; Bogotá-Gregory et al., Reference Bogotá-Gregory, Lima, Correa, Silva-Oliveira, Jenkins, Ribeiro, Lovejoy, Reis and Crampton2020). In addition, how marked distinction in water types affects different components of functional diversity (sensu Mason et al., Reference Mason, Mouillot, Lee and Wilson2005) of Amazonian fish assemblages remains poorly understood.
Our analysis suggests that river colours could be useful proxies of distinctive fish assemblages indicating that anthropogenic disturbance in rivers with contrasting colours could threaten unique fish faunas. Amazonian rivers and their associated floodplains are threatened by innumerous human-drive activities such hydroelectric construction, deforestation and burning of floodplain forests (Castello et al., Reference Castello, Mcgrath, Hess, Coe, Lefebvre, Petry, Macedo, Renó and Arantes2013; Macedo and Castello Reference Macedo, Castello, Oliveira, Maretti and Charity2015; Flores et al., Reference Flores, Holmgren, Xu, Nes, Jakova, Mesquita and Scheffer2017). These alterations in the river channels and their floodplains certainly affect the aquatic biota. These effects on fish assemblages, however, are not fully understood and need to be investigated through long-term monitoring (e.g. Mérona et al., Reference Mérona, Juras, Santos and Cintra2010) in Amazonian rivers with contrasting ecological conditions indicated by their water colours.
Acknowledgements
This publication results from an academic project developed in the Vertebrates I (Fish and Amphibians) class. We are grateful to Universidade Federal do Amazonas (UFAM) for academic support and Fernando Dagosta for assistance during the analysis. Comments of anonymous reviewers contribute to improve the original manuscript. We dedicated this paper to memory of Marcelo Menin for years of dedication to teach vertebrate biology that inspired us as students and teacher.
Conflict of interest
The authors declare that they have no conflict of interest.
Compliance with ethical standards
The authors declare that they following all ethical standards for this journal (Journal of Tropical Ecology).








