Introduction
Knowledge of the distribution and ecology of rare species is critical for their conservation. However, available data on rare species, particularly those that are difficult to identify, are often scarce and may have several shortcomings, including geographical collection bias and high taxonomic error rates (Hamilton et al. Reference Hamilton, Pollino and Jakeman2015). Macrolichens are a good example of this; while they are widely recognized to be excellent environmental indicators (e.g. Giordani et al. Reference Giordani, Brunialti, Bacaro and Nascimbene2012), in many jurisdictions they are poorly documented. As a result, conservation ranks are often assigned tentatively due to insufficient or low quality data (e.g. Goward Reference Goward1996; Government of Alberta 2014). Efforts to fill these knowledge gaps through targeted species-based approaches for lichens alone are often impractical due to limited resources. In this respect, large-scale, non-targeted monitoring is an opportunity to fill such gaps, particularly if such efforts are complemented by rigorous taxonomy and appropriate laboratory techniques. As compared to herbarium collections, geographically extensive, large-scale monitoring initiatives are less likely to suffer from geographical sampling biases because they typically employ random, systematic or stratified sampling, making them more appropriate for analyses of habitat preferences and population dynamics (e.g. Nielsen et al. Reference Nielsen, Haughland, Bayne and Schieck2009). However, large-scale monitoring programmes might not be efficient in providing data on rare or difficult-to-identify species because of a focus on breadth rather than depth of sampling, a resultant de facto emphasis on common species, and reliance on novice technicians conducting rapid assessments (Haughland Reference Haughland2012; Zhang et al. Reference Zhang, Nielsen, Grainger, Kohler, Chipchar and Farr2014). As such, it is important to examine the ability of these large-scale programmes to inform the conservation status of rare and/or taxonomically challenging species.
In this study we examine the ability of a large-scale biodiversity monitoring initiative in Alberta, Canada, to fill the information gap in the distribution and ecology of a rare, taxonomically challenging species, using Cladonia rei as our case study. The Alberta Biodiversity Monitoring Institute (ABMI) is designed around a systematic grid of permanent sites which are surveyed via rapid assessments for human disturbance, habitat metrics, and the occurrence and relative abundance of birds, mammals, oribatid soil mites, vascular plants, bryophytes and macrolichens (ABMI 2010, 2014; www.abmi.ca). Cladonia rei is a fruticose, difficult-to-identify lichen consisting of grey-green, typically sorediate podetia (Brodo et al. Reference Brodo, Sharnoff and Sharnoff2001; Ahti & Stenroos Reference Ahti and Stenroos2013). The morphology of C. rei is variable and podetia may produce secondary squamules, small cups or secondary proliferations (Paus et al. Reference Paus, Daniels and Lumbsch1993; Dolnick et al. Reference Dolnick, Beck and Zarabska2010; Pino-Bodas et al. Reference Pino-Bodas, Burgaz and Martín2010; Ahti & Stenroos Reference Ahti and Stenroos2013). This variability makes C. rei difficult to distinguish from similar, sympatric species, such as C. subulata and C. coniocraea (e.g. Goward Reference Goward1999; Brodo et al. Reference Brodo, Sharnoff and Sharnoff2001; Spier & Aptroot Reference Spier and Aptroot2007), and so relatively inexperienced technicians cannot target it amongst similar subulate Cladonia species. However, C. rei has a reliable chemical trait that can be used to confirm its identity in the laboratory; the presence of homosekikaic acid (Asahina Reference Asahina1938; Østhagen Reference Østhagen1976). This makes C. rei a good case study for the utility of non-targeted monitoring to understand the ecology and distribution of taxonomically challenging species.
The few existing herbarium collections of C. rei from Alberta dating from 1947 suggested the species was broadly distributed but rare (Government of Alberta 2014), while in contrast, ecological studies by Looman (1964 Reference Loomana , Reference Loomanb ) inferred that it could be a common inhabitant of grassland ecosystems. Here we use specimens and related data collected by the ABMI to validate the distribution of C. rei in Alberta, model its distribution using field and remote sensing-derived predictors, including other lichens and vascular plants, and look for predictable community assemblages that co-occur with C. rei. We then reassess its conservation status in the Province, and put the ecology and phenotypic variation of C. rei in Alberta into a global context.
Methods
Study area and survey design
Our study focused on the province of Alberta which covers an area of 661 648 km2 or 7% of Canada’s land mass. Alberta is ecologically diverse, encompassing ecosystems such as alpine and mountain environments, boreal forest, deciduous-dominated dry parkland and mixed-grass and fescue (Festuca) grasslands (Fig. 1, Government of Alberta 2006). We used data and specimens collected by the Alberta Biodiversity Monitoring Institute (ABMI) which samples a core, systematic, 20×20 km grid of 1656 permanent 1 ha sites (Fig. 1, www.abmi.ca), as well as a smaller number of ‘off-grid’ sites targeted to supplement existing environmental and anthropogenic disturbance gradients (Haughland Reference Haughland2012; Burton et al. Reference Burton, Huggard, Bayne, Schieck, Sólymos, Muhly, Farr and Boutin2014). ABMI core sites are grouped into blocks of 9 (3×3) sites, and each block is assigned systematically to 1 of 5 sampling sets with the goal of completing each set in order, given constraints imposed by natural disturbances such as large-scale forest fires, and regional and programme-wide funding. In our study we examined lichen collections from all 778 sites sampled by the ABMI between 2009–2013, representing a diversity of habitat types and anthropogenic disturbances (Fig. 1, Table 1).
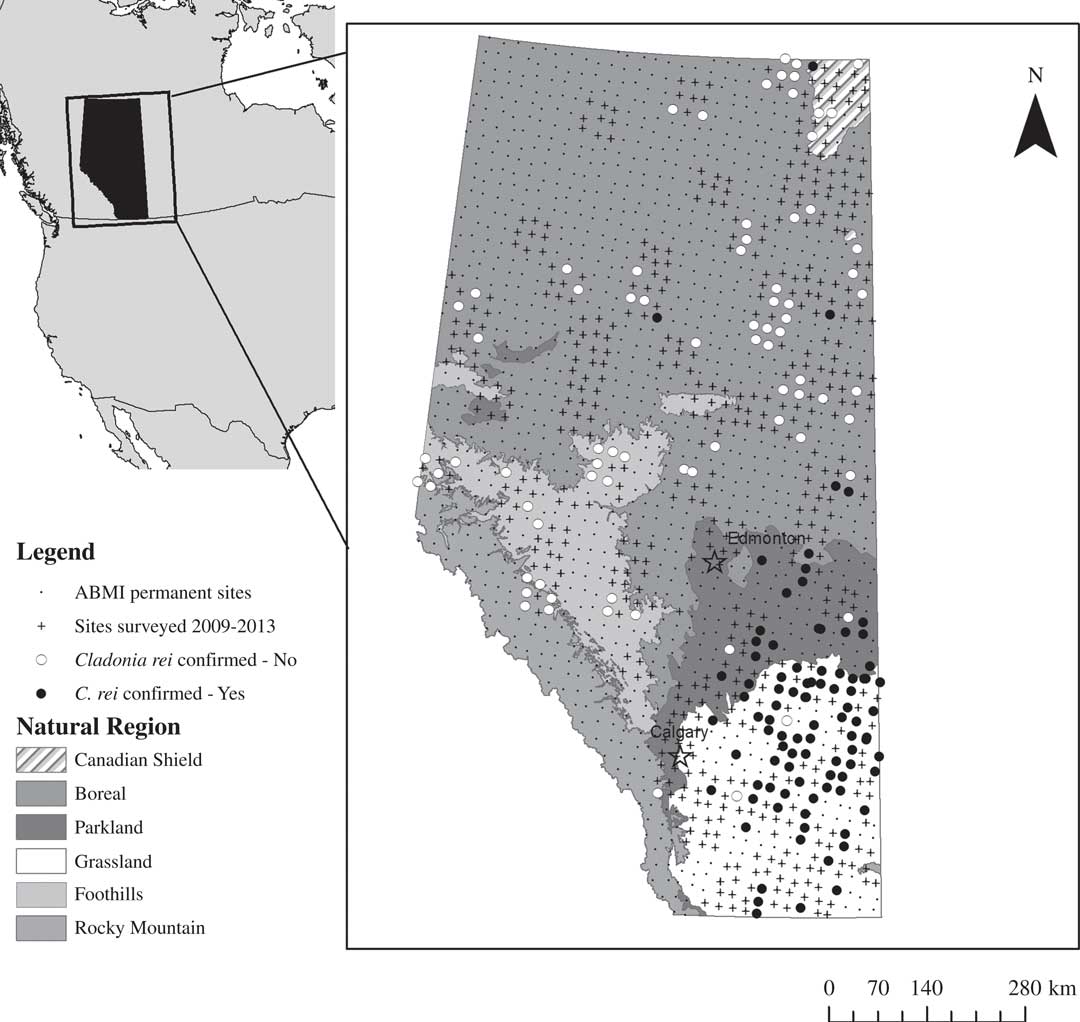
Fig. 1 Cladonia rei distribution in Alberta, Canada resulting from the Alberta Biodiversity Monitoring Institute sampling effort. The map shows all 1656 on-grid ABMI sites, and all ABMI on- and off-grid sites surveyed from 2009–2013. For the latter, sites where C. rei was originally suspected but later determined to be absent are marked with open circles and sites where C. rei was confirmed to be present are marked with closed circles.
Table 1 The Alberta Biodiversity Monitoring Institute (ABMI) is designed around a systematic grid of 1656 sites. Here we show the sampling effort, anthropogenic disturbance samples, and hypothesized and confirmed Cladonia rei detections by natural regions, as represented in the 2009-2013 subset analysed herein.“-” indicates value not applicable

Field methods
Field sampling was conducted by technicians trained by the senior author between May and July of each year, and followed a modified floristic habitat (Newmaster et al. Reference Newmaster, Belland, Arsenault, Vitt and Stephens2005) sampling scheme (detailed methods in ABMI 2010). In each of the 4 quadrants of each 1 ha study site, a 25×15 m plot was established in the outermost corner (Fig. 2). Technicians estimated the amount and type of anthropogenic disturbance per plot. Microhabitats were assigned to 1 of 5 strata (trees, shrubs and vertical structures; logs and stumps; rocks and cliffs; upland and disturbed soils; wetland substrata). At each plot, the collector first spent up to 25 min searching within the boundaries of the plot, collecting unique macrolichen and calicioid morphotypes from microhabitats in the first 3 strata. In each quadrant, the collector then spent 10 min surveying microhabitats and collecting unique macrolichen and calicioid morphotypes from the latter 2 strata on 2 belt transects of 2×25 m (Fig. 2). In total, macrolichen and calicioid samples collected from each quadrant included those from a plot and those from 2 belt transects (a maximum 35 min of survey effort and 5 composite collection bags), and samples from each site included those from all 4 quadrants (a maximum of 2·3 h of survey effort and 20 composite collection bags). All lichen samples were collected for identification in the laboratory. Samples are stored at the Royal Alberta Museum for future accessioning.
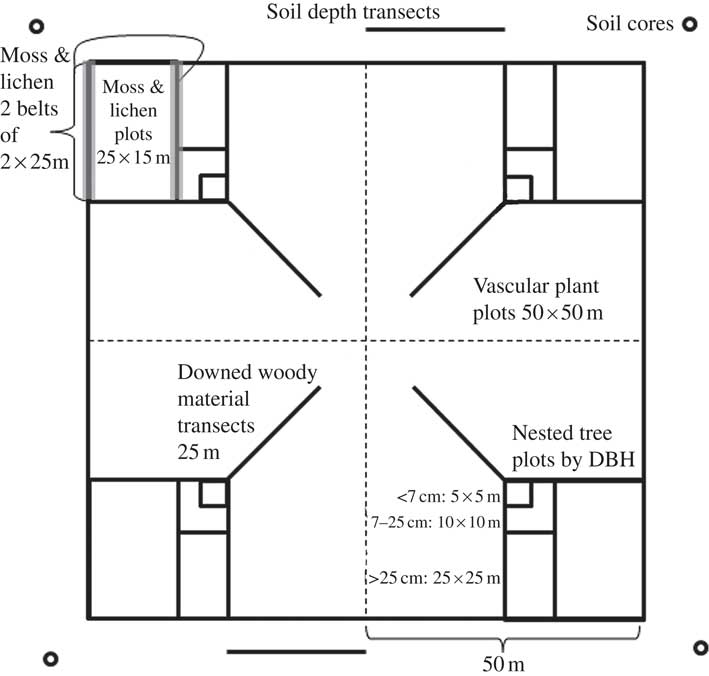
Fig. 2 Overview of lichen collection protocols as well as other key Alberta Biodiversity Monitoring Institute terrestrial protocols, together with their survey dimensions (from 2009 onwards, detailed in ABMI 2010). The 1 ha site is divided into 50×50m squares or quadrants. A summary of the survey protocols is given within the diagram; protocols were repeated in each of the quadrants; soil depth was an exception, it was measured along 2 transects north and south of the 1 ha plot. DBH=diameter at breast height.
Chemical analyses and morphometrics
Spot tests and morphology were used initially to separate putative C. rei from similar Cladonia species. Specimens identified as putative C. rei (PD+ red or PD−, K−, KC−, UV+ dull white or UV−) were examined using thin-layer chromatography (TLC) to confirm the presence of homosekikaic acid (following Orange et al. (Reference Orange, James and White2010), on 10×20 cm plates, and boiling the acetone-specimen mixtures 3 times in a water bath prior to spotting as per I. M. Brodo, pers. comm.). A collection from Ontario was used as the initial standard for homosekikaic acid and fumarprotocetraric acid (1965, I. M. Brodo, 6476 (PMAE)). For 35% of samples we used solvent systems A, B′ and C; however, once it was determined that solvent C was sufficient to distinguish homosekikaic acid from other likely secondary metabolites (such as barbatic or squamatic acid), we typically used solvent C only. Ambiguous results were re-run in all three solvents.
All putative C. rei specimens underwent TLC at the beginning of our study. The results from each round of TLC were used to refine our species concept, our understanding of C. rei’s distribution and to decide which specimens required TLC going forward. During the latter TLC rounds, we analyzed ≥1 specimen from every site within the Grassland and Parklands Natural Regions; if that specimen was confirmed, further TLC was not conducted on putative C. rei from that site. If the representative specimen was incorrect, all additional putative C. rei specimens from the site underwent TLC. In contrast, all putative C. rei specimens from the remaining natural regions of Alberta (mountains, foothills, boreal and shield) underwent TLC.
Using a haphazard selection of verified samples, we recorded the height of the tallest podetium in each sample as well as the presence of primary squamules, secondary squamules, cups, pycnidia, apothecia, secondary proliferations and substratum. Measurements are presented as the mean
![]() $(\bar{x})\,\pm\,{\rm 1}$
standard deviation (SD), followed by the range (smallest–largest observed values) and the sample size (n).
$(\bar{x})\,\pm\,{\rm 1}$
standard deviation (SD), followed by the range (smallest–largest observed values) and the sample size (n).
Additional specimens examined
We located seven accessioned C. rei specimens from Alberta herbaria (ALTA and PMAE). Additional sources searched included: CFB (data provided by G. Pohl in 2013), UBC (UBC Herbarium 2016), UAC (B. Smith, pers. comm.) and the Consortium of North American Lichen Herbaria (2016). All accessioned material of C. rei from ALTA and PMAE (regardless of collection location) was examined and underwent TLC, as did unaccessioned specimens tentatively identified as C. rei from surveys in Alberta and British Columbia with T. Goward and T. Ahti (Supplementary Material Appendix 1, available online). In addition, we examined PMAE collections of Cladonia subulata, C. cornuta, C. coniocraea and C. macilenta (no C. acuminata, C. verruculosa, C. glauca, or C. norvegica from Alberta were accessioned); for each species, a range of specimens chosen to represent the geographical range and morphological variation present also underwent TLC in the three solvent systems.
Habitat variables for modelling
We compiled variables reflecting anthropogenic and natural ecological gradients at small scales (field data) and at larger scales (remote sensing data) to test previously documented ecological patterns for C. rei. Variables for each quadrant were derived from field data and summarized at the site scale by averaging or aggregation (Table 2).
Table 2 Summary of the covariates in Cladonia rei habitat models as well as the ecological arguments for their inclusion. For quantitative data the range, mean and standard deviation are provided; for categorical or binary data the percentages are provided. Where applicable, summaries are presented for both quadrants (Q) and sites (S) where C. rei was confirmed present (181 quadrants, 86 sites) as well as not detected (927 quadrants, 191 sites)

Variables measured in the field
Lichen richness (grouped by substratum affinity: epigeic, both obligate and occasional, and non-epigeic) was included in the model to ascertain whether C. rei was more common in diverse cryptogamic crust communities or depauperate lichen assemblages, possibly behaving as a pioneer (e.g. Osyczka & Rola Reference Osyczka and Rola2013). We included native and non-native vascular plant richness to reflect biotic condition and potentially disturbed soils such as intense grazing in pastures (see Fig. 2, ABMI 2010). Since we observed C. rei growing on Selaginella densa in many of our samples, we included the occurrence of S. densa in our models. Soil pH was also included because Paus et al. (Reference Paus, Daniels and Lumbsch1993) concluded that C. rei was more common on slightly acidic soils (c. 5·9 on average), Looman (1964 Reference Loomana ) on circumneutral soils, while Ahti & Stenroos (Reference Ahti and Stenroos2013) indicate that more basic soils and calcareous rock may be the native substratum of C. rei. We examined total soil organic carbon, which typically decreases when soil has been intensively cultivated and is higher in more fertile, biologically active and moisture retentive soils (e.g. Parton et al. Reference Parton, Schimel, Cole and Ojima1987; Havlin et al. Reference Havlin, Kissel, Maddux, Claassen and Long1990). We also considered soil litter, a proxy for the amount of decomposing organic matter available above the ground. High plant productivity and/or low grazing levels may increase the amount of litter and, concomitantly, diminish established epigeic lichen communities (e.g. Zhang et al. Reference Zhang, Liu, Xu, Xu and Yamanaka2013).
Variables derived from remote sensing
Remote sensing-derived variables are less reflective of fine-scale microhabitats because of their large grain, but large-scale processes can influence species distributions and their inclusion permits models to be mapped across areas not previously surveyed for testing larger-scale habitat associations. We compiled data from geographic information system (GIS) layers for soils, land cover and anthropogenic disturbance (Supplementary Material Appendix 2, available online).
Geographic and climatic variables
Alberta’s categories of Natural Regions and Subregions (Fig. 1; Government of Alberta 2006), in conjunction with latitude and longitude, are good proxies for climate variables and other underlying spatial gradients so we included these as site level variables. We also examined climatic variables estimated from the Alberta Climate Model (Alberta Environment 2005) which reflect average conditions from 1961–1990.
Data Analysis
We conducted logistic regression to relate the occurrence of C. rei to habitat and spatial variables. Analyses considered the presence/absence of C. rei at both site and quadrant scales because each scale represents potentially different ecological processes and thus results might be scale-specific. Of the suite of potential variables representing local habitat conditions, biotic factors (co-occurring flora), climate and geography, we selected 13 habitat variables based on their ecological significance and independence from other variables (Table 2). Variables such as tree density, basal area and canopy cover were excluded after TLC and redetermination but prior to modelling because the realized distribution of C. rei was almost exclusively in the Grassland and Parkland Natural Regions where these variables were not applicable. Other variables were excluded because they were highly correlated (P >0·7). For example, the richness of native vascular plants was highly correlated with total vascular plant richness (r=0·96), as was the richness of lichens with the richness of epigeic lichens (obligate and occasional, r=0·87) and non-epigeic lichen richness (r=0·71). Climate variables were correlated with geography and not considered further; for example, latitude was highly negatively correlated with mean annual temperature (r=−0·95), potential evapotranspiration (r=−0·85) and mean coldest month temperature (r=−0·85), whereas longitude was highly negatively correlated with mean annual precipitation (r=−0·73) and positively correlated with mean warmest month temperature (r=0·7). Prior to analyses, the final set of variables was examined for outliers and checked for collinearity using variance inflation factors (Quinn & Keough Reference Quinn and Keough2002; Zuur et al. Reference Zuur, Leno and Elphick2009). We explored linear and polynomial relationships between occurrence probability and the continuous variables. Accordingly, we incorporated quadratic terms for soil pH and latitude in our multiple regression models. Finally, we assessed the relative importance of each of the predictor variables using standardized regression coefficients in the global model (Schielzeth Reference Schielzeth2010); to obtain the standardized coefficients, continuous predictive variables were standardized as z scores prior to regression. The logistic regression analysis was performed using the glm function in R (version 3.2.2; R Development Core Team 2015).
Species co-occurrence analyses
We used the approach described by Azeria et al. (Reference Azeria, Fortin, Hébert, Peres-Neto, Pothier and Ruel2009) to identify species groups within our lichen and vascular plant assemblages as well as the association of C. rei to those groups. The method applies null-model analysis (Gotelli Reference Gotelli2000) to first obtain the species co-occurrence beyond that expected by chance alone (e.g. a common species will co-occur with many species simply by being more common) and then applies hierarchical clustering to identify species groups. Null models are used to ascertain whether observed patterns of species co-occurrence are beyond those expected if species were randomly distributed (Connor & Simberlof Reference Connor and Simberlof1979; Gotelli & Graves Reference Gotelli and Graves1996; Sanderson Reference Sanderson2000; Azeria et al. Reference Azeria, Fortin, Hébert, Peres-Neto, Pothier and Ruel2009). In this study we employed two types of null models, the fixed–fixed (FF) and the fixed–equiprobable (FE), which have been shown to have reasonably low rates of Type I and II errors (Gotelli Reference Gotelli2000). Both null models maintain species occurrence frequencies from the observation matrix (fixed row totals). The FF also maintains the total number of species at sites (i.e. fixed column totals), while the FE considers sites to be colonized equiprobably (Gotelli Reference Gotelli2000). We applied the two null models because the FF null model effectively reveals segregated co-occurrences (negative associations) but is conservative in detecting aggregated distributions (positive associations; Wilson Reference Wilson1987; Azeria Reference Azeria2004). The converse is the case for the FE null model (Gotelli & Graves Reference Gotelli and Graves1996; Azeria Reference Azeria2004). We thus used the FF and FE null models simultaneously (Azeria et al. Reference Azeria, Ibarzabal and Hébert2012) to detect significant positive and negative associations of C. rei with other lichen species. The null communities (using FF and FE null models) were generated by a quasi-swap algorithm (Miklós & Podani Reference Miklós and Podani2004) using the function ‘permatfull’ in vegan for R (Oksanen et al. Reference Oksanen, Blanchet, Kindt, Legendre, Minchin, O’Hara, Simpson, Solymos, Henry and Stevens2015).
The co-occurrence analysis was conducted at the site scale using species presence/absence data. The plant assemblage was restricted to 249 species occurring in 10 or more sites. As the lichen assemblage was relatively poor, we used a lower threshold (5 sites) to include 63 species or species groups in the analysis. A non-metric multidimensional scaling analysis (NMDS) was carried out on the lichen species matrix to visualize the species groups.
Results
ABMI field surveys resulted in 70 181 lichen specimens, including 27 870 Cladonia specimens. Of those, we identified 435 putative C. rei specimens (Table 1). We analyzed 212 ABMI specimens by TLC, including all putative C. rei samples from the mountain, boreal, foothills and shield Natural Regions (Fig. 1, Table 1). Using the presence of homosekikaic acid (either in the sample or in samples from the same site) we confirmed 293 samples, 93% of which were located in the Grassland and Parkland Natural Regions (Table 1, Fig. 1, Supplementary Material Appendix 1, available online). Most C. rei was found growing on upland soils including upland vegetation and vegetation debris (90% of confirmed samples), followed by rock (6%), wetland substrata (2%), logs and stumps (1%) and on the bases of trees and other vertical substrata (<0·6%). The sole C. rei specimen from the shield Natural Region occurred in undisturbed habitat, while four of the five boreal sites were pastures or adjacent to pastures.
None of the seven accessioned C. rei collections from Alberta were determined to be C. rei (Supplementary Material Appendix 1, available online). Of the c. 200 accessioned Alberta collections of similar species examined (such as C. subulata, C. coniocraea, C. cornuta and C. macilenta), 86 of which underwent TLC, one collection of C. coniocraea was redetermined to C. rei (Supplementary Material Appendix 1, available online). Of the C. rei collections from outside of Alberta, three accessioned C. rei (from Saskatchewan, Iowa and Minnesota respectively) contained homosekikaic acid alone and displayed a similar phenotype to the Alberta material (Supplementary Material Appendix 1, available online).
Chemistry and morphology
We considered the presence of homosekikaic acid as a diagnostic trait; fumarprotocetraric acid was never found as an accessory metabolite. The only accessory metabolite occasionally detected was sekikaic acid. Approximately half of the specimens had detectable fluorescence, typically a dull white, which we found easiest to detect under short-wave (254 nm) rather than longwave light (365 nm). The mean maximum podetium height was 16±9 mm (range 2–58 mm, n=72; Fig. 3). The podetia rarely proliferated or proliferated very sparingly (16% with proliferations, n=86 for all further morphometrics; Fig. 3A–C), sometimes resulting in forked tips but almost never giving rise to cups with subulate marginal proliferations (Fig. 3C). Specimens rarely became scyphose; 11% of podetia ended in a narrow or funnel shaped cup. The lower third to half of the podetia were typically grass green and corticate, with farinose to granular patchy brown and green soredia forming towards the terminus (Fig. 3D–H). Primary squamules were often persistent (67%) and secondary squamules were common on the lower half of the podetia (66%; Fig. 3D–H & L). Relatively large apothecia, as depicted in Brodo et al. (Reference Brodo, Sharnoff and Sharnoff2001), were never found and small, inconspicuous apothecia or pycnidia were observed in 35 specimens (41%). The ABMI specimen from the northern Canadian Shield Natural Region was phenotypically consistent with C. rei confirmed in this study from eastern North America, British Columbia and Europe, albeit lacking fumarprotocetraric acid (Supplementary Material Appendix 1, available online).

Fig. 3 Representative Cladonia rei collections from Alberta. The grassland ecotype most commonly encountered is depicted in samples D–H and L, with unbranched podetia and secondary squamules. The cupped or proliferating phenotypes depicted in A–C are extremely rare, as are the non-squamulose, almost entirely sorediate forms depicted in I–K. Scales=1 cm. ABMI collections pictured: A, on Selaginella densa, Grassland Natural Region (GNR), 2015, site 1458, 51·0°N, −112·1°W, 600355; B, on S. densa, GNR, 2012, site 1368, 51·6°N, −110·8°W, 84639; C, on S. densa, GNR, 2014, site 1503, 50·5°N, −111·1°W, 428022; D, on organic debris, GNR, 2012, site 1506, 50·4°N, −110·3°W, 69394; E, on S. densa, Parkland Natural Region, 2011, site OG-ABMI-1090-1, 53·4°N, −111·5°W, 306021; F, on soil, GNR, 2012, site 1395, 51·3°N, −110·3°W, 84652; G, on soil, GNR, 2009, site OG-ABMI-1341-1, 51·6°N, −110·6°W, 154859; H, GNR, 2012, site OG-ABMI-1498-1, 50·7°N, −112·5°W, 90376; I, on soil, GNR, 2010, site 1500, 50·6°N, −111·9°W, 251534; J, on soil, Boreal Forest Natural Region (BFNR), 2014, site 922, 54·3°N, −111·9°W, 652277; K, on soil, BFNR, 2013, site OG-ABMI-561-1, 56·5°N, −115·0°W, 76165; L, on S. densa, GNR, 2013, site 1519, 50·4°N, −112·0°W, 15214. Numbers reference unique records in the ABMI lichen database, latitudes and longitudes are hazed to within a 5·5 km radius of the actual site to protect site locations.
Habitat models
Similar coefficient estimates in magnitude and direction resulted from both scales of analysis (Tables 2 & 3). Here we present the site results; while the quadrant model might represent habitat at a more lichen appropriate scale, the habitat models for site level analyses are more conservative and samples are independent. Variables measured in the field, remotely derived habitat variables and geographical variables all had significant predictive capacity. The occurrence of C. rei was higher at more epigeic lichen rich sites, with each additional species detected increasing the likelihood of detecting C. rei by a factor of c. 2·7 (Tables 2 & 3). Cladonia rei was more common where Selaginella densa was recorded, supporting our observations in the laboratory. While native vascular plant richness was on average twice as high at sites with C. rei (Table 2), neither native nor non-native richness co-varied significantly with C. rei occurrence. Cladonia rei was more likely at sites with more extensive litter cover. It also occurred more often at grassland sites with more productive soil types, probably in part because it was also detected predominantly in pastures, typically areas utilized for their productivity. The probability of detecting C. rei in a pasture site were 16 times greater than that of detection in a site dominated by alienating disturbances (which alter the soil long term, including cultivated fields, industrial activity, linear features such as powerlines and roads, and human settlements). Undisturbed or ‘intact’ sites were also more likely to contain C. rei than sites with disturbance but the effect was not as strong (10 times more likely) or significant at α=0·05 (Table 3). Cladonia rei exhibited a quadratic relationship with soil pH, occurring at intermediate but slightly acidic pH levels; however, the mean difference in pH between occupied and unoccupied sites was small (Tables 2 & 3).
Table 3 Summary of models estimating the probability of detecting Cladonia reiat a site (α=0·05, df=235, pseudo r 2=62%) and a quadrant (df=1066, pseudo r 2=47%): variables presented are model coefficients (ß), standard errors (SE), z-score values, P values (Wald z statistic) and percent change in odds ratios per unit increase in significant covariates (%ΔOR). Alienating disturbances include those that alter the soil over the long term and include industrial disturbances, crop fields, residential and urbanized areas, and linear features such as roads and railways. The superscript 2 indicates quadratic variables included to account for polynomial relationships

Within the regions modelled, C. rei was more likely to be found at intermediate to high latitudes, which corresponded to lower mean annual temperatures, colder winters and lower evapotranspiration. Geographically, C. rei was more likely to be found in the Northern Fescue Natural Subregion of the grasslands, a zone that transitions to the Parkland Natural Region to the north. We found no relationship between C. rei presence and longitude, that is the gradient represented by the Rocky Mountains region with its colder mean monthly temperatures and higher mean annual precipitation in the west and the drier, more arid grasslands to the east. Additionally, there was no relationship between C. rei presence and soil total organic carbon. Figure 4 represents the extrapolated final model of C. rei distribution based on landscape level variables.
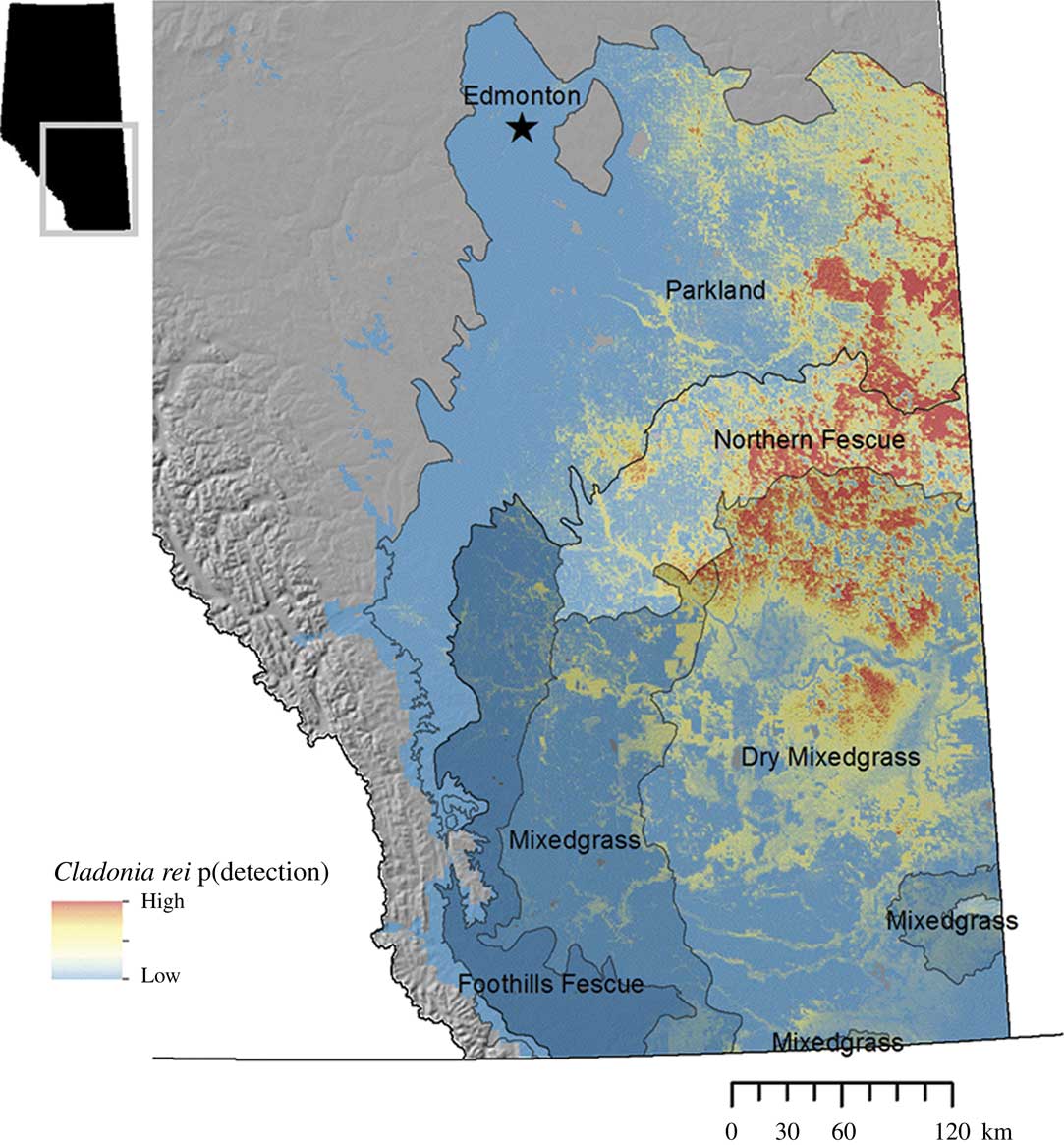
Fig. 4 Final site-scale habitat model for Cladonia rei, based on the landscape-level remotely-derived and geographic variables that could be extrapolated across space. p (detection) is the model-predicted probability of detecting Cladonia rei, from high (close to 1, coloured red) to low (close to 0, coloured blue). Model extrapolation is limited to the Grassland and Parkland Natural Regions of Alberta. Regions are labelled as either the Natural Region (Parkland) or Subregions within the Grassland Natural Region (all other labels).
Species associations
Three groups emerged from our analyses of lichen co-occurrence (Fig. 5, Supplementary Material Appendix 3, available online). Group 1 was largely composed of epiphytic and epixylic species common on deciduous Populus balsamifera, P. tremuloides, coniferous Picea glauca and downed wood, and included some epigeic and epixylic Peltigera species. Group 1 was also the most stable grouping and membership was largely robust to analytical methods. Group 2 and 3 species were more loosely structured and in general members of each group showed more positive than negative associations with each other. Group 2 contained a diverse assemblage of grassland genera, including vagrant and semi-vagrant Cetraria and Xanthoparmelia species and Phaeophyscia constipata, together with epigeic Cladonia, and epilithic Rhizoplaca and Xanthoria species. Group 3 was dominated by generalist epigeic and occasionally epixylic Cladonia and Peltigera species, as well as Cetraria arenaria. Cladonia rei clustered with group 3 under both null models (Supplementary Material Appendix 3, available online, Figs 5 & 6). Within Group 3 the species with the strongest associations with C. rei were C. cornuta ssp. cornuta, C. gracilis ssp. turbinata, C. multiformis, C. subulata, C. chlorophaea and C. pocillum.
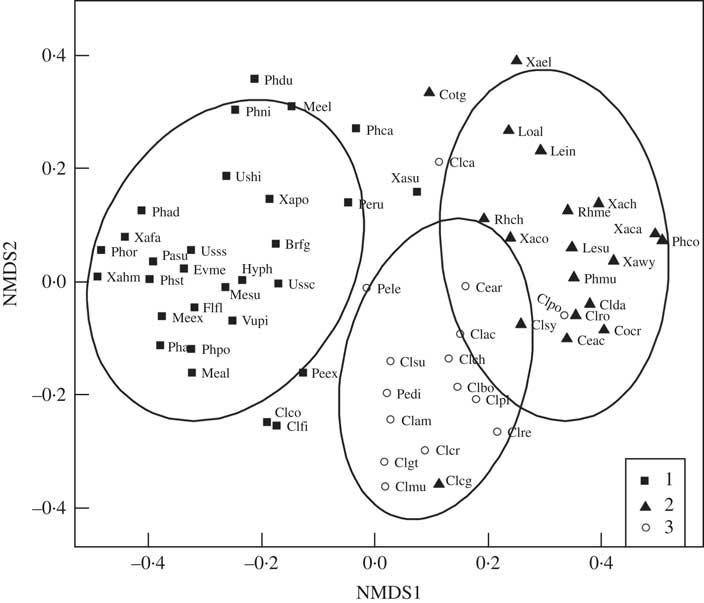
Fig. 5 Non-metric multidimensional scaling ordination plot of associations between Cladonia rei and other lichens from southern Alberta, as determined by the site-scale species co-occurrence analyses. Symbols are as follows: group 1 (squares), mainly epiphytic lichens common on Populus balsamifera, P. tremuloides and Picea glauca; group 2 (triangles), grassland genera including vagrant and semi-vagrant Cetraria and Xanthoparmelia, epigeic Cladonia epilithic Rhizoplaca and Xanthoria; group 3 (circles), generalist epigeic and occasionally epixylic Cladonia (including C. rei) with Peltigera species and Cetraria arenaria. Brfg - Bryoria fuscescens grp. (includes cf. vrangiana), Ceac - Cetraria aculeata, Cear - Cetraria arenaria, Clac - Cladonia acuminata, Clam - Cladonia arbuscula ssp. mitis, Clbo - Cladonia borealis, Clca - Cladonia cariosa, Clch - Cladonia chlorophaea s.s., Clcg - Cladonia chlorophaea grp. (suite of PD-, granular sorediate species including C. grayi, C. merochlorophaea), Clco - Cladonia coniocraea, Clcr - Cladonia cornuta ssp. cornuta, Clda - Cladonia dahliana (psoromic acid chemotype of C. symphycarpa), Clfi - Cladonia fimbriata, Clgt - Cladonia gracilis ssp. turbinata, Clmu - Cladonia multiformis, Clpl - Cladonia pleurota, Clpo - Cladonia pocillum, Clre - Cladonia rei, Clro - Cladonia robbinsii, Clsu - Cladonia subulata, Clsy - Cladonia symphycarpa, Cocr - Collema crispum s. lat., Cotg - Collema tenax grp. (mostly infertile), Evme - Evernia mesomorpha, Flfl - Flavopunctelia flaventior, Hyph - Hypogymnia physodes, Lein - Leptogium intermedium, Lesu - Leptogium subtile grp. (including L. subtile, cf. nanum and L. tenuissimum), Loal - Lobothallia alphoplaca, Meal - Melanelixia albertana, Mesu - Melanelixia subaurifera, Meel - Melanohalea elegantula, Meex - Melanohalea exasperatula, Pasu - Parmelia sulcata, Pedi - Peltigera didactyla, Peex - Peltigera extenuata, Pele - Peltigera lepidophora, Peru - Peltigera rufescens, Phpo - Phaeocalicium populneum, Phco - Phaeophyscia constipata, Phni - Phaeophyscia nigricans, Phor - Phaeophyscia orbicularis, Phad - Physcia adscendens, Phaa - Physcia aipolia & alnophila, Phca - Physcia caesia, Phdu - Physcia dubia, Phst - Physcia stellaris, Phmu - Physconia muscigena, Rhch - Rhizoplaca chrysoleuca, Rhme - Rhizoplaca melanophthalma, Ushi - Usnea hirta, Ussc - Usnea scabrata, Usss - Usnea substerilis & subfloridana, Vupi - Vulpicida pinastri, Xafa - Xanthomendoza fallax, Xahm - Xanthomendoza hasseana & montana, Xaca - Xanthoparmelia camtschadalis, Xach - Xanthoparmelia chlorochroa, Xaco - Xanthoparmelia coloradoensis, Xasu - Xanthoparmelia subdecipiens, Xawy - Xanthoparmelia wyomingica, Xael - Xanthoria elegans, Xapo - Xanthoria polycarpa.
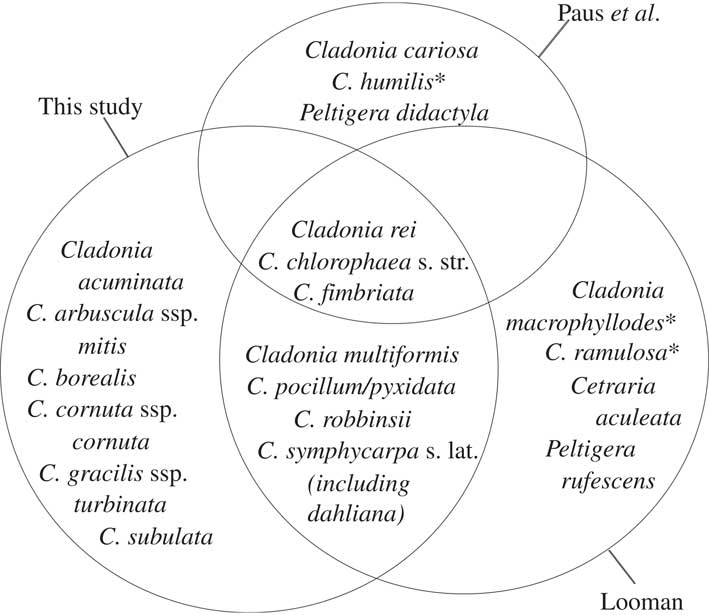
Fig. 6 Lichen species found to be positively associated with Cladonia rei in this study compared with those of Looman’s phytoassociation Cladonietum nemoxynae (1964 Reference Loomana ) for the grasslands of Saskatchewan, Canada and the Cladonietum rei of Paus et al.(Reference Paus, Daniels and Lumbsch1993) for anthropogenic habitats in Central Europe. Species within all three circles are associates in all three studies, while those included in a single circle are associates in one study only. *=species absent from ABMI’s collections.
Five groups emerged from our analyses of vascular plant co-occurrence with C. rei (Table 4, Supplementary Material Appendix 3, available online). Cladonia rei clustered with group 1, which was a mixed ecological group including species common in mesic fescue grasslands, deciduous parklands and dry pine (Pinus spp.) forests and included low shrubs (e.g. Rosa woodsii, Symphoricarpus occidentalis), grassland species (e.g. Festuca hallii, Carex inops, Thermopsis rhombifolia) and boreal/parkland transition plants (e.g. Achillea millefolium). Group 2 was characteristic of native dry mixed grasslands further south, and included Artemisia spp., Selaginella densa and many grassland specialists (e.g. Opuntia polycantha, Gaura coccinea). Group 3 represented cultivated fields with agronomic species such as canola (Brassica rapa) and wheat (Hordeum vulgare), and weedy communities found in disturbed areas (e.g. Matricaria discoidea, Capsella bursa-pastoris), whereas group 5 contained grassland plants and exotic weeds common in the margins of prairie and saline wetlands (e.g. Beckmannia syzigachne, Taraxacum officinale, Rumex crispus). Group 4 appeared to be a geographical grouping, with parkland species (e.g. Agrimonia striata), upland exotics (e.g. Cirsium arvense) and native plants (e.g. Lathyrus venosus) found in the dry boreal subregions. At the individual species level, we found positive associations between C. rei and most plants in groups 1 and 2, negative associations with most group 3 plants, and very few significant associations with group 4 and 5 plants (Supplementary Material Appendix 3, available online).
Table 4 Vascular plants significantly positively or negatively associated with Cladonia rei in both co-occurrence models (see Supplementary Appendix 3). Asterisks (*) indicate species recognized by Looman (1964Reference Loomana, Reference Loomanb) as associates of the Cladonietum nemoxynaephytoassociation in Saskatchewan grasslands

Conservation status
Using the NatureServe Status Calculator (2015), we calculated a rank of “apparently secure” for C. rei in Alberta. The rank of “apparently secure” is a technical rank of S4 on a 5 point scale ranging from S1 (critically imperilled) to S5 (secure), versus the then-assigned rank of S2. The metrics used in that calculation were as follows: area of occupancy of 100–500 4 km2 grid cells, number of occurrences equal to 81–300, population size of 2500–10 000 (assuming a minimum of 25 thalli per occupied site based on field observations), many occurrences with excellent or good viability, moderate generalist with some key requirements scarce, an overall low threat impact due to its ability to persist with the dominant disturbance in its range (cattle-grazing), and the low likelihood that a large percentage of current pasture land will be converted to crops in the next decade.
Discussion
Our study suggests that large-scale monitoring employing novice collectors, combined with rigorous taxonomy, can redress pseudo-rarity due to geographically biased sampling for at least some taxonomically challenging species. With >50 000 accessioned lichen collections in herbaria, we confirmed one Cladonia rei from Alberta. Conversely, samples from large-scale monitoring resulted in almost 300 collections, largely from the south-eastern quarter of the province. In combination with TLC, the ABMI samples improved our understanding of the ecology and habitat preferences of a taxonomically challenging species. These data show that C. rei is neither acting as a pioneer species nor commonly associating with industrial or anthropogenically altered habitats in Alberta. Instead, the habitat models and species co-occurrence analyses show that C. rei occupies an intermediate niche in pastures and grasslands of the deciduous parkland of central Alberta and the mixed grass and fescue grasslands found to the south, as originally inferred by Looman (1964 Reference Loomana , Reference Loomanb ). Below we explore these conclusions in more detail.
Tackling pseudo-rarity
Previous studies have shown that non-targeted data collection by novices can provide robust community level information (McCune et al. Reference McCune, Dey, Peck, Cassell, Heiman, Will-Wolf and Neitlich1997). Here we show that these initiatives can also provide valuable species level data, even for taxonomically challenging species that require chemical verification in the laboratory. ABMI specimens show that C. rei is not imperiled in Alberta as herbarium collections suggest (Government of Alberta 2014), but rather is common where it occurs. For C. rei, the disparity between the perceived and actual abundance is in part due to the tradition of exclusive reliance on validated reports and specimens in herbaria to assess status. Agencies are often challenged to incorporate resources such as ecological research or industrial environmental impact assessments unless collections are accessioned (Whitehead et al. Reference Whitehead, Lane, Haughland and Caners2015). Thus, the widespread presence of C. rei (as Cladonia nemoxyna) in the grasslands apparently first observed by Looman (1964 Reference Loomana , Reference Loomanb ) while studying the neighbouring province of Saskatchewan, could not be considered because no Alberta collections were accessioned. Similarly, it is challenging for conservation agencies to incorporate ABMI data in part because of the lag between reporting and accessioning collections (this lag is common across large-scale monitoring programmes, such as the Forest Inventory and Analysis Lichen Indicator in the United States, USDA Forest Service 2017). It is vital that these programmes ensure specimens are conserved and available for verification and inevitable future taxonomic revisions to realize their full value in informing conservation.
Documenting species’ distributions and informing ecology
Geographically unbiased, large-scale sampling can provide more complete ecological gradients to test species habitat associations. Most studies within the boreal and temperate biomes show C. rei to be an occupant of anthropogenic habitats such as lawns, gravel piles, road and railway embankments and industrial areas (Ahti & Stenroos Reference Ahti and Stenroos2013; Osyczka & Rola Reference Osyczka and Rola2013; Rola et al. Reference Rola, Osyczka and Nobis2014; but see Looman 1964 Reference Loomana , Reference Loomanb ). The large-scale monitoring dataset used here is the first to show C. rei to be more abundant in undisturbed grasslands and native pastures than in concurrently surveyed industrial and heavily altered anthropogenic habitats. In fact, the area occupied by C. rei coincides closely to a region characterized as ‘High Value Landscape’, or HVL, by the Prairie Conservation Forum in Alberta (ABMI 2015). The HVL has 2–3 times less anthropogenic disturbance than the area outside the HVL and is managed with the priority of preserving large patches of native vegetation and biodiversity corridors (ABMI 2015).
One hypothesis that could reconcile previous studies with our findings is that dry deciduous forest edges and grasslands are the native habitat of C. rei and anthropogenic habitats most closely approximate those conditions; however, some aspects of its distribution remain puzzling. For example, despite surveys across many disturbed sites, it is unclear why C. rei is apparently absent or rare in anthropogenic habitats in Alberta. It is also not apparent why it is absent from other grassland ecosystems in North America (e.g. the Columbia Basin grasslands of Washington and Oregon (Looman 1964 Reference Loomanb ; Hammer Reference Hammer1995; McCune & Rosentreter Reference McCune and Rosentreter2007; Root et al. Reference Root, Miller and McCune2011) or the interior grasslands of southern British Columbia (Goward & Ahti Reference Goward and Ahti1997; Goward Reference Goward1999; UBC 2016)). Assuming these issues are not due to taxonomic discrepancies, more data on the species’ distribution and habitat affinities across its southern range (e.g. Macaronesia, East Africa, Asia and New Zealand; Archer & Bartlett Reference Archer and Bartlett1986; Goward Reference Goward1999; Ahti & Sohrabi Reference Ahti and Sohrabi2006; Ahti & Stenroos Reference Ahti and Stenroos2013; Ahti et al. Reference Ahti, Sohrabi, Davydov, Pino-Bodas and Stenroos2016) might help answer these questions. In the interim, we hypothesize that C. rei is absent from more southerly, open, arid grasslands because it has higher moisture requirements than many other grassland macrolichens and it has the ability to adapt to lower light levels. For example, C. rei is one of only a small number of lichen species the first author has observed persisting under thick, dead grass in grassland grazing exclosures.
The large-scale, multi-taxon dataset presented here also provided the opportunity to test previous studies of C. rei associations. Groupings in data-driven classifications are contingent on the degree of heterogeneity in the data, the pool of species included, the completeness of the data and the spatial scale of the study (e.g. Legendre & Legendre Reference Legendre and Legendre1998), so differences between studies are expected. It is of interest therefore that our results support the phytosociological findings of Looman in nearby temperate grasslands (1964a, b) more than 50 years after his surveys and despite disparity in the survey scale. Of the “recognition species” Looman (1964 Reference Loomana ) commonly found with C. rei that were also recorded by ABMI, all but two were positively associated species in our co-occurrence analyses (Fig. 6). Our co-occurring species also overlap in part with Paus et al.’s (Reference Paus, Daniels and Lumbsch1993) Cladonietum rei association, common in disturbed habitat in Central Europe (Fig. 6), but aside from C. rei, the shared species largely behave as generalists in our region (see http://species.abmi.ca/pages/species/lichens.html for distribution maps of lichen species, based on ABMI collections).
Taken together, our data suggest that rather than acting as a bare soil pioneer in Alberta, C. rei occurs more commonly with a relatively rich epigaeic lichen community, including Cladonia fimbriata, C. chlorophaea, and C. robbinsii (Fig. 6). In Alberta, this community occurs primarily in the south-eastern grasslands with slightly acidic soils (supporting the earlier findings of Paus et al. Reference Paus, Daniels and Lumbsch1993) that are relatively productive and overlain with litter, as well as on Selaginella densa mats.
Grassland ecotype
In Alberta, the majority of C. rei specimens fit what we have tentatively called the grassland ecotype, characterized by the absence of fumarprotocetraric acid, cups or apothecia as well as sparse proliferations, a persistently corticate base, a persistent primary thallus and typically abundant secondary squamules. Considered individually, these traits have been documented by other authors (e.g. Paus et al. Reference Paus, Daniels and Lumbsch1993; Goward Reference Goward1999; Ahti & Stenroos Reference Ahti and Stenroos2013). The review of herbarium specimens and literature from North America and Europe (Fig. 3, Supplementary Material Appendix 1, available online), highlighted characteristics of C. rei commonly documented outside of our study area but rarely observed in the ABMI collections. These include 1) presence of fumarprotocetraric acid (60% in Pino-Bodas et al. (Reference Pino-Bodas, Burgaz and Martín2010) and 53% Paus et al. (Reference Paus, Daniels and Lumbsch1993) vs. 0% here), 2) sparse secondary squamules and an evanescent primary thallus (35% with basal squamules in Pino-Bodas et al. (Reference Pino-Bodas, Burgaz and Martín2010) vs. 67% here), 3) brownish soredia to the base of the podetium (38% of specimens with a corticate base in Paus et al. (Reference Paus, Daniels and Lumbsch1993)), 4) cups (forming “frequently and even on young podetia” in Ahti & Stenroos (Reference Ahti and Stenroos2013) vs. 11% here), and 5) well-developed apothecia present in part (e.g. Brodo et al. (Reference Brodo, Sharnoff and Sharnoff2001) vs. absent here). The grassland ecotype may be the result of development in the relatively shady, moist boundary layer of grassland vegetation with abundant litter, within an otherwise arid, grassland environment. The persistence of primary squamules and relatively abundant secondary squamules may optimize photosynthetic area in such habitats. It is unclear why fumarprotocetraric acid is absent from C. rei within our study area.
Potential sources of error and unexplained variance
There are potential challenges when making autecological inferences from coarse-scale biodiversity surveys. The scale of ABMI surveys is 100–1500 times larger than other studies of C. rei communities (ABMI methods: 375 m2 per plot, 100 m2 per belt transect, for a total area surveyed of 1700 m2 per site vs. 0·1–1·0 m2 in Looman (1964 Reference Loomana ) and Rola et al. (Reference Rola, Osyczka and Nobis2014)). Larger plots capture more microhabitats and consequently more species, but they also capture more ecological variation and may obfuscate fine scale phytosociological or ecological patterns. ABMI sites are placed without regard to the homogeneity of the environment whereas most ecological studies place plots in homogeneous patches of vegetation. Depending on how the mean patch size of a landscape compares with ABMI’s survey area, this can introduce additional variation. A further information gap is the under-representation of the mountain Natural Region in the ABMI dataset (Table 1, Fig. 1). Historically, lichenologists have surveyed this region but survey effort likely remains an issue given that traditional haphazard surveys often don’t include all meso- and microhabitats. While all samples of putative C. rei from Alberta’s mountains were redetermined as other Cladonia species (Supplementary Material Appendix 1, available online), further sampling is required. Detection error is also likely to be unequal across sites; however, it is not likely to be driving our results given that technicians had similar levels of experience and that overall lichen detection is probably lowest in the grasslands where most C. rei collections were made (D. L. Haughland, pers. obs.). Finally, there is likely to be some error in our site classifications; for example, some undisturbed grassland sites might have been grazed or over-seeded historically even though the site lacks visually perceptible or remotely sensed signals of disturbance at the time of the survey.
Recommendations
Many lichen collectors do not have ready access to TLC; it may be one of the major factors impeding taxonomic accuracy for lichenologists without laboratories. Some herbaria routinely conduct TLC on incoming challenging genera such as Cladonia but they appear to be in the minority as only 4% of C. rei specimens had TLC data in the CNALH (2016). Solutions include TLC services from a few central laboratories at a low per specimen cost; more work on spot test development for a wider variety of lichen substances (e.g. FeCl3 test for homosekikaic acid (Huneck & Yoshimura Reference Huneck and Yoshimura1996)); more resources for herbaria so they can accession and TLC more material; adoption of a qualifier, such as ante TLC, or some analogous system to indicate specimens in taxonomically challenging groups identified without TLC, allowing that uncertainty to be incorporated into conservation ranking.
Wider use of standardized, geographically unbiased sampling would probably be beneficial in testing many ecological assumptions across longer ecological gradients; however, our aim is not to be prescriptive. Instead we outline some practices that have benefited the ABMI lichen programme. Key amongst these is the adoption of a standardized sampling method across all habitat types, focusing on effort per unit area and a standardized set of microhabitats. It is a basic premise yet one that is often challenged, even in a rapid survey, because technicians search both depauperate and biodiverse microhabitats in order to document absence as well as presence. The standardized plot size was ascertained experimentally using accumulation curves of occupied microhabitats rather than species (Haughland Reference Haughland2012). Lastly, we have sacrificed detailed information for individual collections to increase field sampling efficiency so that the method fits within the suite of protocols conducted by the ABMI. These practices do not negate observer effects however, and the percentage of the total species pool sampled by each technician varies (see also McCune et al. Reference McCune, Dey, Peck, Cassell, Heiman, Will-Wolf and Neitlich1997). The benefit of inclusion within a larger monitoring programme is access to sites across the entire province and access to supporting ecological information.
Conclusion
Understanding the spatial distribution of rare or common species is critical because of the insight it lends to studies of dispersal, gene flow, speciation rates, adaptive plasticity, threats to species from climate change or other anthropogenic activities, biodiversity richness, hotspots and conservation prioritization. North America has received more survey effort than many regions but gaps still remain in our understanding of lichen ecology and distribution (e.g. Goward & Ahti Reference Goward and Ahti1997). Programmes such as the ABMI are one tool to narrow these knowledge gaps. The ABMI is unique in North America because of its systematic sampling of a diversity of habitats (both forested and unforested) and anthropogenic activities. However, programmes that share design elements exist in other areas, including the United States, United Kingdom, Switzerland, New Zealand, parts of Australia and South America (e.g. reviewed in Haughland Reference Haughland2012; Herzog & Franklin Reference Herzog and Franklin2016). These programmes can circumvent comparative methodological issues, geographical sampling biases, and with concerted effort, taxonomic uncertainty, that otherwise might confound both meta-analyses and smaller scale studies documenting species autecology and assessing conservation status.
We thank Mireille Martel and Darcie Thauvette, an essential part of the lichen team at the Royal Alberta Museum. We are grateful to the scientists and agencies that make the data used herein freely available by bringing the ABMI to realization (including Stan Boutin, Dan Farr, Jim Schieck, Kirk Andries, Tyler Cobb and Jim Herbers), as well as to the dozens of field staff that conduct ABMI surveys each year. We thank Varina Crisfield for her expertise in the interpretation of the vascular plant species co-occurrence analyses, Teuvo Ahti for alerting us to the work of J. Looman, Trevor Goward, Peter Crittenden, Chris Ellis and two anonymous reviewers for constructive comments on drafts of this manuscript, and the herbaria that allowed us access to their collections.
Supplementary materials
To view supplementary material for this article, please visit https://doi.org/10.1017/S0024282918000099












