Article contents
The Structure of the Atom
Published online by Cambridge University Press: 03 November 2016
Extract
Had I been called upon to address the Association a few years earlier on a subject of this nature, my task would have been much more difficult. Model atoms were then becoming almost as numerous as the sands of the sea. The very simplicity of the quantitative phenomena shown by the simplest atoms was an obstacle to progress. For example, the simple spectrum of Hydrogen,— the so-called Balmer series,—consisting of a set of frequencies given by the formula
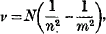
where n and m take all possible integral values, was somewhat too simple if considered by itself instead of in relation to phenomena of a widely different type. And as a consequence, we had at least five completely different forms of suggested structure of a Hydrogen atom, each of which, by suitable mathematical artifices, could be made to yield such a set of frequencies.
Information
- Type
- Research Article
- Information
- Copyright
- Copyright © Mathematical Association 1922
- 2
- Cited by

