The Guinea Worm Eradication Programme (GWEP) offers a fascinating but neglected case study for historians of medicine. Though useful practitioner histories of guinea worm exist, there is little historiography of the disease or its eradication programme beyond six pages in Nancy Stepan’s Eradication: Ridding the World of Diseases Forever? Footnote 1 Nevertheless, the GWEP provides a valuable window both on the workings of eradication and the interactions of biological and historical forces in shaping medical landscapes. A particular confluence of historical forces – principally the primary healthcare (PHC) movement – and the biological nature of guinea worm – the only disease transmitted exclusively by drinking water – forced the World Health Organisation (WHO) to develop a novel method of eradication, which focused on PHC and changing behaviours around water. This shaped how medical practitioners involved in the WHO viewed guinea worm (Dracunculus medinensis), as they came to define it as a problem of health education, a problem of PHC, a problem of cross-border mobility, and, most recently, a problem of zoonosis (that is, transmission of disease from animals to humans). Guinea worm’s transformation into a zoonosis further demonstrates the power of eradication programmes to shape not only social but also biological natures of disease. Under pressure from the GWEP, guinea worm was, in Chad, able to evade eradication by developing an entirely novel ecology based around a new host: domestic dogs.
Guinea worm disease (dracunculiasis/dracontiasis) is painful and debilitating, with victims temporarily (and sometimes permanently) disabled by the worm emerging from their body, usually in the leg or foot. This is rarely fatal, but secondary infections can kill or necessitate amputation. No anti-dracunculiasis drug or vaccine is currently available – worms are extracted from the body by hand – and it is hoped that it will not only be the second human disease eradicated, but also the first eradicated without a drug or a vaccine (that is, without a specific technology of treatment or prevention).
In biological terms, the first-stage guinea worm larvae inhabit stagnant water, where they are ingested by the copepod crustaceans I will refer to as cyclops. For much of its history Cyclops was considered a single genus, but it was subdivided in the late twentieth century, with several of the new genera (particularly Metacyclops, Thermocyclops and Mesocyclops) known to be important hosts of Dracunculus. Footnote 2 I here use a deitalicised and decapitalised cyclops as convenient term for the group. The first-stage guinea worm larvae grow inside the cyclops through two further larval stages before water containing the cyclops is ingested by humans. Stomach acid dissolves the cyclops but not the larval worms, which, making their way into the body cavity, mature and mate there, with the males dying off while the females gestate. After a year, the female moves towards the surface of the body, emerging through a blister. When the victim enters water, the female emerges and disgorges its larvae, and the lifecycle begins again.
Dracunculiasis once occurred from the Aral Sea to the Caribbean, but ecological change and successive eradication programmes, firstly in Bukhara (1923–31), later in Tamil Nadu (1959–1980), and finally globally (1986–present; GWEP) have left it nearly eradicated, with only 27 cases recorded in 2020, 15 in 2021, and 6 in the period January–July 2022.Footnote 3 Following Tamil Nadu’s guinea worm programme, India’s national Ministry of Health and Family Welfare launched its own eradication programme in 1983.Footnote 4 The World Health Assembly endorsed the elimination of dracunculiasis in 1986 and its eradication in 1991; for this reason, Stepan considers the eradication programme to begin in 1991, but I use 1986 as a beginning of practical work to eradicate dracunculiasis on a global scale.Footnote 5
Guinea worm disappeared from the Caribbean sometime in the nineteenth century.Footnote 6 It was eradicated from Central Asia by 1931, from South Asia by 1999 and Arabia by 2000 (Yemen was the only known endemic country in Arabia by the twentieth century).Footnote 7 Since then, it has been found in a gradually contracting band of West, Central and East Africa. As of 2022, only Chad, Ethiopia, Mali and South Sudan remain endemic, with Angola (last reported case 2020) and Sudan (last reported case 2013) in the precertification stage and Cameroon (last reported case 2020) in the certification stage of eradication.Footnote 8 While this paper will cover some developments in India, the geographic focus of its sources and the current distribution of guinea worm necessitates a focus on West Africa and the Sahel.
Information on the disease burden of dracunculiasis is hard to come by; before the global eradication programme dracunculiasis was under-recorded and incidence estimates were consequently imprecise, but in 1986 incidence was estimated at 3.2 million new infections annually.Footnote 9 The 2016 Lancet Global Burden of Disease study estimated guinea worm’s global burden at 50.7 (95%; confidence interval 35.3–69.2) thousand Disability-Adjusted Life Years (DALYs) in 1990, falling by 100% to less than a hundred DALYs in 2016.Footnote 10 In the same period, this study estimated the burden of malaria to fall from 60 389.3 thousand DALYs to 56 201.2 thousand DALYs; schistosomiasis 2096.8 to 1863.6 thousand DALYs; intestinal nematode infections 7460.9 to 3331.2 thousand DALYs, and onchocerciasis 1420.4 to 962.5 thousand DALYs. It should be emphasised, however, that guinea worm eradication had already made considerable progress by 1990. In 2017, Christopher Fitzpatrick et al. estimated that guinea worm eradication had averted between one and two thousand DALYs of disease weekly compared to no intervention.Footnote 11 However, they note that no disability weights for modelling DALYs specific to guinea worm disease have been devised.Footnote 12
In recent years, historians of medicine have increasingly emphasised the roles of actors outside of transnational institutions, empires and superpower states in eradication and other global health achievements. For instance, to challenge the general view of smallpox eradication as an American-driven by-product of Cold War geopolitics, Sanjoy Bhattacharya and Carlos Campani emphasise the earlier role of Latin American nation-states, while Erez Manela underlines the crucial role of the USSR, as do Marcos Cueto, Theodore Brown and Elizabeth Fee.Footnote 13 Dora Vargha likewise challenges a bipolarity-focused historiography by discussing the poliomyelitis policies of the smaller Warsaw Pact governments.Footnote 14 Steven Palmer’s study of hookworm similarly locates the ‘peripheral origins of international health’ in Latin American states, while Socrates Litsios explores how the concept of PHC emerged from a variety of locations including China, Yugoslavia and South Africa.Footnote 15 Other histories have emphasised the agency of local actors in negotiating and shaping health policy, such as Ben Walker’s detailed account of missionaries, colonial administrators, local chiefs and national politicians in Ghana.Footnote 16
This paper will follow in this tradition and use the GWEP to explore developments in global health policy on an international scale, while also emphasising how local actors – including populations of guinea worm – realigned global policy on a local level. The GWEP offers many examples of local agency shaping global health; the emphasis placed by the GWEP on PHC and volunteers facilitated considerable local variation in the forms the GWEP took. I use WHO publications to examine how the GWEP conceptualised guinea worm, and how responding to the challenges of dracunculiasis shaped its approach to eradication.Footnote 17 To expand my view of the GWEP beyond an institutional history of the WHO, I use the memoirs of Luke Edungbola, Zonal Facilitator for dracunculiasis eradication in northwest Nigeria, focusing particularly on his accounts of actors other than international organisations and nation-states, as well as published medical and social science papers.Footnote 18 As I will show, the GWEP varied significantly across time and space; Edungbola’s memoirs reflect only the specific context of northwest Nigeria (as well as the perspective of a professional physician), but this in itself provides a valuable local corrective to the larger-scale perspectives of the central WHO archive. Similarly, I use social science studies, which are highly situated in the specific areas of their fieldwork, offering an alternative perspective to that of international organisations. I view these sources as complementary, each illuminating different facets of the programme. Additionally, I use twenty-first-century scientific literature on dracunculiasis to contextualise the changing scientific and medical problem to which the WHO was responding.
I argue principally that the GWEP represents a novel approach to eradication. This approach was horizontal more than vertical, participatory more than top-down, driven by local people more than international organisations. It was created both by historical circumstance and the unique epidemiology and ecology of guinea worm. Initially a development project, the GWEP became increasingly focused on PHC through the 1990s, as the World Bank pivoted away from water projects.Footnote 19 As the twenty-first century opened, the GWEP focused more closely on cross-border transmission, and it increasingly emphasised health education and individual prophylaxis through technologies such filters and practices of safe drinking. Through the 2010s, guinea worm’s changing ecological relationships forced the WHO to recontextualise it as a zoonosis and invest in vector control in addition to PHC and health education measures aimed at shaping human behaviour. Part 1 of this paper examines the novelties of a low-tech participatory, PHC-focused approach to eradication in depth, and notes how this approach emphasised the agency of local, ordinary people over that of medical professionals and transnational institutions. Part 2 explores two specific challenges to the GWEP that came to prominence in the twenty-first century: cross-border transmission of guinea worm and its transformation into a zoonotic disease. Throughout, I will explore the different ways in which guinea worm has been conceptualised and problematised by global health, emphasising the importance of local actors in shaping global health.
Part 1: the other road to eradication
Histories of global health often make use of a division between a technological, biomedical and vertical paradigm of health, focusing on narrow biomedical interventions against specific diseases, and a social, horizontal paradigm, which focuses on the social determinants of health, PHC, and generalist preventative medicine. Though this division has often been challenged, it continues to offer a useful framing device for historians: Randall Packard’s History of Global Health, for example, is dedicated to explaining the apparent dominance of the technological and vertical, while Cueto, Brown and Fee chronicle the conflicts between the two paradigms within the WHO.Footnote 20 Eradication programmes are generally taken, based on the examples of the Malaria and Smallpox Eradication Programmes, to belong to the realm of the technological, biomedical and vertical.Footnote 21 Though Stepan notes that the GWEP was profoundly different than the classical model of eradication – a low-tech eradication programme focused on PHC and community participation – she treats it as the exception that proves the rule of the top-down nature of eradication.Footnote 22 Similarly, the story of eradication – as told by Stepan, Packard, and Cueto, Brown and Fee – is often told as the story of institutions, an understanding that again is not fully applicable to the community- and individual-focused GWEP.Footnote 23
This section, after outlining some key differences between the GWEP and earlier eradication programmes, explores two elements of the GWEP that were largely absent from earlier eradication programmes – PHC and health education – before discussing how different interventions, principally filtration (safe drinking) and case containment, emphasised the agency of different actors. This complicates the role of the WHO as an institution and demonstrates that crucial work in eradicating dracunculiasis was done by residents of endemic areas themselves. The ecology of guinea worm and the demands of the historical moment forced a new approach to eradication, which has proved successful and which challenges the notion that eradication programmes are necessarily narrow, vertical and technological. Eradication can focus on social determinants of health such as clean water provision and individual behaviour, and it can be leveraged to build networks of PHC.
The different mode of eradication created by the GWEP prioritised participation of individuals and communities in endemic regions above technological interventions. Whereas previous eradication efforts had often succeeded and failed by their technologies – vaccination and insecticide – guinea worm eradication was decidedly low-tech, using the ancient technologies of wells and filters.Footnote 24 This denotes a fundamental shift in agency from technologies and institutions to individuals and communities.
The GWEP was a product of the 1980s. Spurred by successes against the disease in India and ‘the special opportunity afforded by the International Drinking Water Supply and Sanitation Decade [1981–1990] to combat dracunculiasis’, it, as Cueto, Brown and Fee have identified, had a distinctively post-Alma-Ata focus on both PHC and health education.Footnote 25 The GWEP was in many respects a development project (in the post-1970s sense of community-based development), aiming to bring safe water sources, networks of health surveillance, PHC capacity and health education to isolated and often impoverished communities.Footnote 26 Consequently, guinea worm was defined as a development problem, a PHC problem and a health education (that is, behaviour) problem. However, some of the development aspects fell away over time, leaving a GWEP focused more on behaviour and PHC than clean water provision by the late 1990s.
The reasons for these focuses were both historical and biological. Dracunculiasis’s status as ‘the only disease which is transmitted exclusively through drinking water’ meant that provision of safe water – a classic development project – was a simple and highly effective intervention.Footnote 27 One strategy, pioneered in India, was to give priority funding for water projects to endemic villages.Footnote 28 This blurs the line between horizontal and vertical intervention as, even though it was targeted at a single disease, a supply of safe water prevents many diseases. Likewise, health workers trained to treat guinea worm typically performed other health work and allowed other health campaigns to ‘piggyback’ on the networks of trained volunteers created by the GWEP, further complicating any vertical/horizontal divide.Footnote 29 ‘Integrating’ guinea worm work with other health work became a controversial issue in Global Health from the 1990s, but in practice, it was acknowledged that Village Volunteers worldwide were already performing more general PHC work.Footnote 30 This further illustrates the considerable latitude that Volunteers working in isolated areas could have in shaping their own practice, as well as the medical authority that they could derive from their position – and implies a broader lack of PHC provision in dracunculiasis-endemic areas.
From the beginning, the GWEP was defined as a development project, justified by ‘the considerable adverse effects of dracunculiasis…on health, agriculture, education and the quality of life in affected areas’.Footnote 31 In this framework, guinea worm was defined as a development problem, a threat to education and agriculture (as victims could neither work nor walk to school). This view of the disease only strengthened; by 1991 the World Health Assembly was…
…deploring…the continuing adverse effects of dracunculiasis on health, including that of mothers and children, as well as its constraining effects on agriculture, sustainable development and education in endemic areas…Footnote 32
Cases of guinea worm tend to be evenly distributed across genders, if not skewed towards men; this resolution should therefore be read as part of a more general engagement at the WHO with both post-natal healthcare and the language of development.Footnote 33 WHO documents throughout the GWEP frequently refer to the economic and educational effects of dracunculiasis.Footnote 34 Retrospectively justifying his involvement in eradication, Luke Edungbola similarly remarks upon the ‘socioeconomic consequences’ of dracunculiasis and the ‘complications, misery, impoverishment, underdevelopment, shortened life-expectancy and death’ that it could bring.Footnote 35 Michele Barry, noting criticism of single-disease programmes, has even defended the GWEP as ‘leaving a legacy of development’.Footnote 36
Primary healthcare at the end of the road
PHC and eradication are often seen as antithetical.Footnote 37 But by the time the GWEP began in 1986, PHC was viewed as the future, an antidote to the excessively technological interventions of the past, and a gateway to a more patient-centred medicine.Footnote 38 The GWEP therefore became an eradication programme focused on PHC, and guinea worm became defined as a PHC problem.
The pathology of dracunculiasis meant that three main elements of PHC – education, prevention and treatment – were intertwined. By the time victims of most diseases seek treatment, they have had ample opportunity to pass on their infection. But with guinea worm, the most distinctive and visible symptom – the blister and the emerging worm – is also the key means of transmission. Prompt extraction of the worm massively reduces the risk of a victim contaminating local water sources. Having a PHC worker nearby to respond to cases as quickly as possible was therefore crucial. Treatment as Prevention is generally taken to be a twenty-first century innovation triggered by the usefulness of antiretrovirals against Human Immunodeficiency Virus (HIV), and Allan Brandt even argues that this caused a fundamental breakdown of the line between treatment and prevention in global health.Footnote 39 (Guillaume Lachenal, however, notes that the concept has many antecedents in colonial medicine).Footnote 40 The biology of guinea worm, though, forced the WHO to engage with the mixed nature of treatment and prevention even before it was acknowledged as such.
Guinea worm’s occurrence in isolated locations, in places where water programmes had not reached (and, after the 1980s, were unlikely to reach) and unmapped villages ‘at the end of the road’ challenged the WHO’s new focus on PHC.Footnote 41 Furthermore, the combination of a long incubation period and the need for immediate treatment meant that PHC workers had to be embedded in communities over long periods of time. A smash-and-vaccinate approach like that adopted in the closing stages of the smallpox campaign was not feasible.Footnote 42 Nor was relying entirely on existing healthcare workers, who might be based many miles from immobile dracunculiasis patients. Both the disease and the historical moment demanded a PHC worker in every village. Thus was, in the 1990s, the Village Volunteer born. Perhaps inspired by China’s ‘Barefoot Doctors’ and drawing from longer traditions of volunteer health work (particularly in West Africa), the Village Volunteers would be a crucial part of the GWEP, with their duties combining treatment, prevention and education.Footnote 43
The form of the Village Volunteer programmes varied by country and region, again illuminating varying regional understandings of PHC and the agency of national and regional governments as well as local communities and individual Volunteers in shaping the GWEP. In Burkina Faso an existing network of volunteer health workers (something many countries did not possess) were trained in guinea worm treatment and prevention.Footnote 44 Nigeria required its Volunteers to be literate, but Ghana found illiterate Volunteers equally reliable.Footnote 45 By 1996, 80% of Mauritanian Village Volunteers were women, while Burkina Faso trained both a man and a woman in each village.Footnote 46 Likewise, in several regions such as northern Ghana, it was necessary to train different Volunteers from the multiple ethnic groups living in the same area.Footnote 47 This too illustrates the flexible pragmatism of the GWEP and the ways in which local actors were able to adapt a global programme to local circumstance.
PHC has been constantly shifting and evolving, as different actors remake it according to their own priorities.Footnote 48 Local movements, including those supported by international (eg., missionary or Rockefeller Foundation) networks in Ghana, South Africa, Yugoslavia and China, had long anticipated the global PHC movement of the 1980s which birthed the GWEP and local actors continued to remake PHC long after the global movement faltered.Footnote 49 The GWEP similarly created a particular guinea worm-focused form of PHC that would continue to be practised by Village Volunteers long after Selective Primary Healthcare and Structural Readjustment had dimmed the bright ambitions of the 1980s.Footnote 50 For this reason, ongoing debates about the nature of PHC intrude surprisingly little into the documentation surrounding Village Volunteers, which is narrowly focused on the specific guinea-worm-centric mode of PHC that the WHO and the Volunteers were creating.
This form of PHC practised by the Village Volunteers was largely preventative; a 2000 training manual for Sudanese Volunteers states ‘the main purpose of a village volunteer is to prevent transmission in every case’ and lists in detail the means, including house-to-house searches, recording and reporting cases, and educating their neighbours, by which Volunteers were hoped to achieve this.Footnote 51 Treatment, as alluded to, was prevention-focused; Volunteers extracting worms were taught to take their patient’s history, which aimed not to identify the ailment, but to locate infected water sources.Footnote 52 Even in 1985, before the global programme had begun, the WHO advised that ‘the main effort of the PHC worker in the control of guinea-worm disease should be…preventing infection.’Footnote 53
An important aim of PHC is to have health workers who are familiar with their patients and their culture embedded in communities. The year-long incubation of dracunculiasis, and the fact that it required changing behaviours around water, meant that it was crucial for the Village Volunteers to retain the trust of their neighbours. The training manual emphasised respectful and friendly behaviour, instructing volunteers to…
…not enter the house unless you are invited; Obey the customary rules…Do not be critical: you are there to help solve the guinea worm problem.Footnote 54
The contrast with Paul Greenhough’s famous 1995 paper on coercion in smallpox eradication rather suggests the WHO was keen to show it had learned the importance of good relations with communities.Footnote 55 The Village Volunteer not only had to live among their patients but also teach them how to prevent the disease, something that required their trust.Footnote 56 Likewise, Volunteers needed people to trust them enough to come to them to treatment, which was also important for surveillance and prevention. Volunteers were informed:
If you listen and show respect, community leaders will come to respect and trust you, and people will feel comfortable talking to you.
Get to know your community…talk to community leaders and members including the elders, women’s groups, men’s groups, religious leaders, traditional healers, and agricultural workers. Let people know who you are and explain what you are doing; Discuss how you can work together to prevent and eliminate guinea worm.Footnote 57
Eradicating a disease as tenacious as dracunculiasis required the participation of the entire community. The recruitment of locals who were familiar with the customs and mores of their neighbours helped in this regard, as well as ensuring that Volunteers could respond quickly to cases in their own locality. Edungbola remarks that external consultants ‘often jeopardized the good relationship between the Nigerian field staff and their communities’ via ‘inflammatory comments’, ‘probably due to lack of experience or due to ignorance of the socio-cultural peculiarities and political sensitivity in the areas of operation’.Footnote 58 A level of cultural knowledge was required, which foreigners might lack.
This local knowledge could also provide challenges. For instance, the Yoruba of Oyo State, Nigeria, had a conception of sobia that imperfectly corresponded to biomedical understandings of dracunculiasis.Footnote 59 While the Yoruba sobia was in some respects more clinically detailed than biomedicine’s dracunculiasis, even distinguishing two different kinds of pain resulting from the worm’s emergence (the normal blunt pain of sobia and the more peppery pain of sobia eleta), it also included symptoms, such as the feeling of the worm moving beneath the skin (sobia awoka), which were foreign to biomedicine, and had a profoundly different conception of its aetiology.Footnote 60 Nevertheless, ‘participatory training’ of Volunteers was able to transcend these differences by creating a consensus that recording should focus on the emerging worm, as this was the stage when the worm was most dangerous to the community.Footnote 61 It would be easy to use this case to frame local knowledge of guinea worm as a static obstacle for health workers to overcome; but it instead shows how local knowledge systems can be flexible and changing, and how these can be valuable aids to health education, providing a basis for shared understandings of health and disease to be created. Indeed, following this breakthrough, case reporting by the Village Volunteers was found to be more accurate (in biomedicine’s terms) than by Local Government Area enumerators.Footnote 62 A participatory approach to guinea worm eradication, reliant on volunteer primary health workers trusted by their neighbours could result in better surveillance than that provided by health professionals alone.
Volunteers had to earn the trust of their neighbours; but communities also had to be encouraged to trust their Volunteer. A 1999 educational comic aimed at children depicts its two protagonists running to fetch the Village Volunteer when their friend from another village manifests a guinea worm.Footnote 63 The Volunteer is represented as ordinary (dressed casually and engaged in agriculture, Figure 1) but authoritative, instructing the children and their parents on how to prevent dracunculiasis.Footnote 64 He is depicted throughout with a first-aid kit by his side.Footnote 65 Sadly, there is no readily available evidence of how this comic was received, but it remains a uniquely useful record of how the WHO conceived of Village Volunteers and how the WHO wanted guinea worm and the GWEP to be perceived. The ideal Volunteer, in the view of the WHO at least, became a local leader, known and trusted by their community, a paragon of PHC.
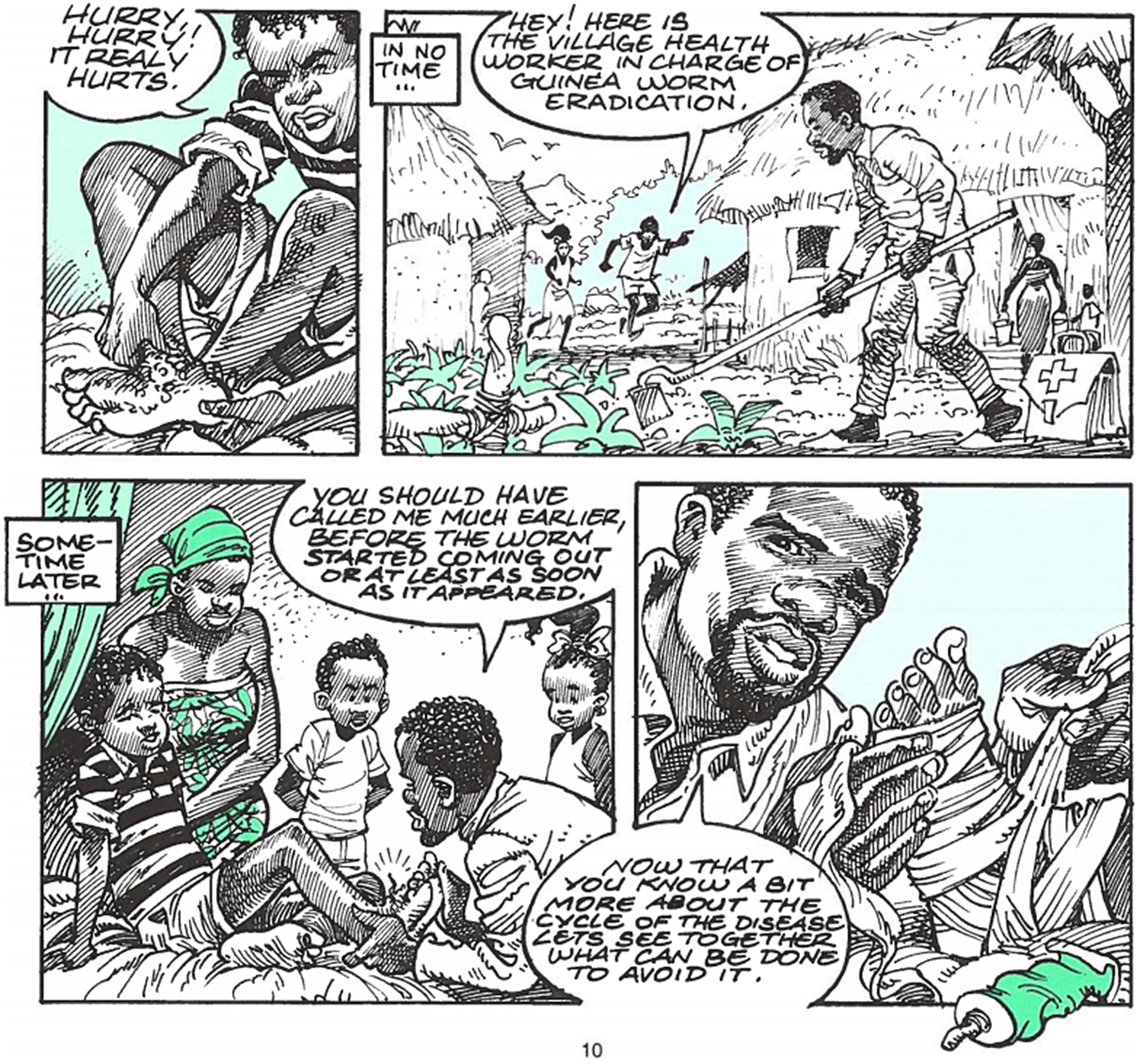
Figure 1 The Village Volunteer; WHO/CDS/DRA/99.2, 10.
The notion that an eradication programme could rest in the hands of village-based volunteers was a victory for PHC, as was the very idea of a PHC worker in every village. Unlike previous eradication programmes, the low-tech nature of the GWEP meant it could be entrusted to individuals with little training, while the nature of the disease necessitated the programme be embedded in communities. The result was a novel approach to eradication, which put PHC foremost, and – despite the support of the WHO, the Carter Centre and UNICEF – meant eradication can be said to have been achieved by local laypeople as much as by professional public health workers.Footnote 66 The GWEP was not the first or only use of volunteer health workers as, for example, Walker discusses in the case of missionary medicine in Ghana, many countries, particularly in West Africa, had existing traditions of volunteerism in health.Footnote 67 Similarly, Deepak Sobti and Marcos Cueto note the importance of Rotary International volunteers in eradicating poliomyelitis from Peru in the late 1980s and early 1990s.Footnote 68 Nevertheless, the GWEP was unusual in making volunteers and PHC such a central and essential part of eradication. It is now a cliché that guinea worm will be the first disease ‘eradicated without vaccines or drugs’, but perhaps it will also be the first disease eradicated by PHC workers embedded in communities.Footnote 69 And, as noted above, a crucial part of the work of these Volunteers was health education.
Health Education
One reason why it was so important for Village Volunteers to be trusted, and another indicator of the participation-focused nature of the GWEP, was their role in health education. It is clear in WHO documentation that this was euphemism for encouraging people to behave in ways conducive to what the WHO defined as health, thus defining dracunculiasis as a behavioural problem. Just as HIV became a problem of persuading people to practise safe sex, so guinea worm increasingly became a problem of persuading people to practise safe drinking.Footnote 70 This allowed individuals with no medical training to participate in eradication, but it also reflected that governments were often simply unable to provide basic securities such as safe water. An increasing emphasis on education and PHC into the 1990s and the twenty-first century may consequently reflect the World Bank’s retreat from safe water projects.Footnote 71
As early as 1985, the WHO regarded health education as the PHC worker’s ‘most important sphere of influence’ in relation to dracunculiasis.Footnote 72 Once the GWEP began, Village Volunteers, alongside other health educators, such as teachers, encouraged their neighbours to rely on safe water, filter suspect water and keep infected persons out of the water.Footnote 73 Encouraging correct maintenance and use of filters was also a priority.Footnote 74
Village Volunteers seem to have embraced their role in education: a 2021 study of Chad, despite identifying concerning knowledge gaps, uneven coverage and equipment shortages, found that most residents learned about guinea worm from Volunteers and GWEP staff, and those living in villages visited more often by Volunteers had a significantly better knowledge of the disease.Footnote 75 The Village Volunteer training guide, meanwhile, under the section ‘Health Education’, includes a series of pictures illustrating the narrative of infection, telling a memorable story about where guinea worm comes from – and like many stories, this one is peppered with moral lessons such as ‘if you have guinea worm, keep away from the water’.Footnote 76 The purpose of education was to change behaviour.
Health education was not a one-way street, however. The importance of incorporating local understandings of the disease (in this case Sobia) into Volunteer training was noted by William Brieger and Carl Kendall, while Sandy Cairncross, Eka Braide and Sam Bugri describe villagers negotiating with GWEP by refusing to engage with health education unless water supplies (which they knew to be a better guarantee of health) were on the table.Footnote 77 Education aimed to change behaviour, but communities were not passive recipients of either education or behaviour change. Effective health education, as well as effective surveillance, required community participation and engagement, something repeatedly noted by social scientists and health professionals alike.Footnote 78
The WHO International Certification Teams (ICTs) therefore made a point of noting whether they saw awareness-raising posters around villages and interviewing residents of at-risk areas to ascertain how far knowledge about guinea worm had percolated.Footnote 79 Edungbola reports a wider variety of tactics employed in northwest Nigeria:
Massive and sustained public enlightenment campaigns were launched from the national level to the grass roots level, using radio stations, television, theatres, mosques, churches, town criers (village announcers), markets, schools, sporting events and festivals to promote health education. Posters, surveillance booklets and other literature materials written in English were translated into Hausa and Ajamin…Footnote 80
This seems to have been successful – in 2003, WHO reported:
One of the most effective interventions has been health education with the aim of motivating communities to use safe water sources, or, where these are not available, to use simple cloth or nylon filters…Health education has also been successful in persuading communities to prevent persons with emerging worms from having contact with water sources…volunteers have also played an instrumental role in health education of communities.Footnote 81
The effectiveness of health education was measured by whether it changed behaviour. Meanwhile in 2013, outside the WHO, the Journal of Paediatrics and Child Health ran an editorial entitled ‘Changing behaviour to eliminate the Guinea Worm’, which cheerily asserted that ‘eradication will be achieved by changing behaviour’.Footnote 82 Given the range of behaviours that residents of endemic areas were expected to exhibit, it seems that they did as much to eradicate guinea worm as their healthcare workers.
Locating Agency
The GWEP seems a kaleidoscopic mix of personal, community and state-led interventions, in contrast to the standard historiographical emphasis on state and institutional action. Though focuses shifted between these elements over time, from its inception the GWEP was planned to involve ‘provision of safe drinking water sources, active surveillance, health education, vector control, and personal prophylaxis’ – a broad mix of collective and individual interventions.Footnote 83 To eliminate guinea worm from whole communities, or at least to radically reduce its transmission, new wells could be sunk, or, as was common practice in India, existing wells could be rebuilt to preclude wading into them.Footnote 84 However, these are expensive, labour-intensive processes, which also require suitable geology and materials.Footnote 85 In addition, either the state or the community, or another actor with capital (and access to labour), must actively initiate these projects. As Christian McMillan has pointed out, since the late 1980s, the World Bank – one of the most important actors with capital in global health – has been increasingly reluctant to do this.Footnote 86
Filtration is cheaper and more reliable than safe water provision, and it requires only individual action, but it only protects the individual. Filtration to prevent dracunculiasis is an old practice, but India began experimenting with funnel filters in 1981, and filters had become an important part of the GWEP in Madhya Pradesh by 1989, while the WHO sponsored studies on filters in 1991 and 1997, and encouraged filter use in their educational materials.Footnote 87 The WHO’s increased focus on filters in the 1990s presumably resulted from declining international funding for water projects.Footnote 88 Individuals were encouraged to filter water whenever they collected it – and the eradication process involved surveys to see if they did.Footnote 89 Different preventative technologies were available in different situations – nomadic groups, those living in areas with unsuitable geology, or where no money was available for well-building had to rely on filters. But the simplicity of filtration belies the fact that encouraging individual prophylaxis rather than collective interventions is a distinctively recent development in public health, and it may reflect a wider shift from communitarian to individualist thinking in health.Footnote 90 However, promoting filtration is also a tacit admission that governments cannot provide safe water; it must be noted that the debt burden imposed on many African states was considered by both the WHO and UNICEF to be a major obstacle to financing PHC and the GWEP in the 1990s.Footnote 91 Filtration, like Volunteer schemes, is an effective measure well-suited to cash-poor countries and communities; but it must be acknowledged that this poverty was an externally imposed barrier limiting the options of those at risk from dracunculiasis.
There is a similar question of agency regarding containment. When someone manifests a worm, whose responsibility is it to keep them out of the water? Is it the state’s (‘the government must contain cases’), the individual’s (‘if infected, you must keep out of the water’) or the community’s (‘we must keep infected people out of the water’)? Even if an individual knew to stay on dry land, arrangements still had to be made within their community for water to be brought to them by someone else. WHO documentation often implies that the state should contain cases, but in practice, different places reached their own solutions.Footnote 92 Edungbola reports that in northwest Nigeria:
…individuals and communities were empowered to…apply all the preliminary interventions immediately (eg., prevent the infected person(s) from entering and contaminating the community source(s) of drinking ponds…).Footnote 93
He also records how, when the Local Government Area Chief Executive refused to act on a dracunculiasis outbreak, a Korowa community posted their own guards to prevent anyone from entering the ponds.Footnote 94 Communities as well as individuals and governments could institute case containment. In some areas this may have been the only feasible option: Edungbola describes villages that his teams were ‘the first government people’ ever to visit and quite literally put on the map.Footnote 95
Community-led self-help leaves little trace in the archives of transnational institutions, but Edungbola records a variety of actors, from children to the women of a village acting in an organised bloc, leading anti-guinea-worm efforts.Footnote 96 A 1993 study of Mali, moreover, identified three crucial ‘favourable factors’ influencing uptake of both knowledge and filters (that is, changed behaviour): ‘villager’s motivation, social cohesion and the influence of returning migrant workers’.Footnote 97 The study notes that the villagers of Karena and Sogomela solicited help in eradicating guinea worm ‘despite the short period of [educational] intervention’.Footnote 98
Communities could also adapt their own institutions to combat guinea worm: Cairncross, Braide and Bugri, for instance, report a variety of innovations.Footnote 99 In Cote d’Ivoire, villages switched from a pay-by-bucket to a universal subscription model to raise funds for pump maintenance, incentivising the use of safe water; in Ghana village women imposed their own fines on those entering the village pond with an open lesion; and in Burkina Faso, a novel village office, the bouilla naba (chief of the pond) was instituted and entrusted with maintaining and guarding village ponds.Footnote 100
In their study of Burkina Faso, Vinh-Kim Nguygen et al. have identified how participating in HIV groups and antiretroviral treatment became means by which HIV-positive individuals could ‘self-fashion’ and create a ‘therapeutic citizenship…a set of rights and responsibilities’, which also provided membership to both local and international communities.Footnote 101 Detailed research would be needed on how individuals and groups conceived of their role in guinea worm eradication to assess how far Nguygen’s concepts are applicable to the GWEP, but many of these local innovations created novel sets of rights, responsibilities and roles surrounding water. This allowed a cohesive village to self-fashion a village-level citizenship in the form of actions taken at an individual and community level to protect themselves and their neighbours from guinea worm.
Indeed, the WHO was keen to portray the GWEP as African- and community-led. In the educational comic the villagers (led by their Volunteer) announce ‘we have to get organised’; ‘together, let’s find solutions for the village that we can handle ourselves’; and finally, ‘we must participate by doing the things described in this booklet’ (Figure 2).Footnote 102 ‘We must’ recurs frequently, and every character, from the lab-coated scientists to the local elders, is recognisably African, and most wear African styles of dress, whether traditional or more casual. The audience is meant to recognise themselves in the comic and act accordingly. As the Village Volunteer programme also demonstrates, this was a programme that aspired to work from the ground up, demonstrating that eradication programmes are not inherently ‘top-down’.
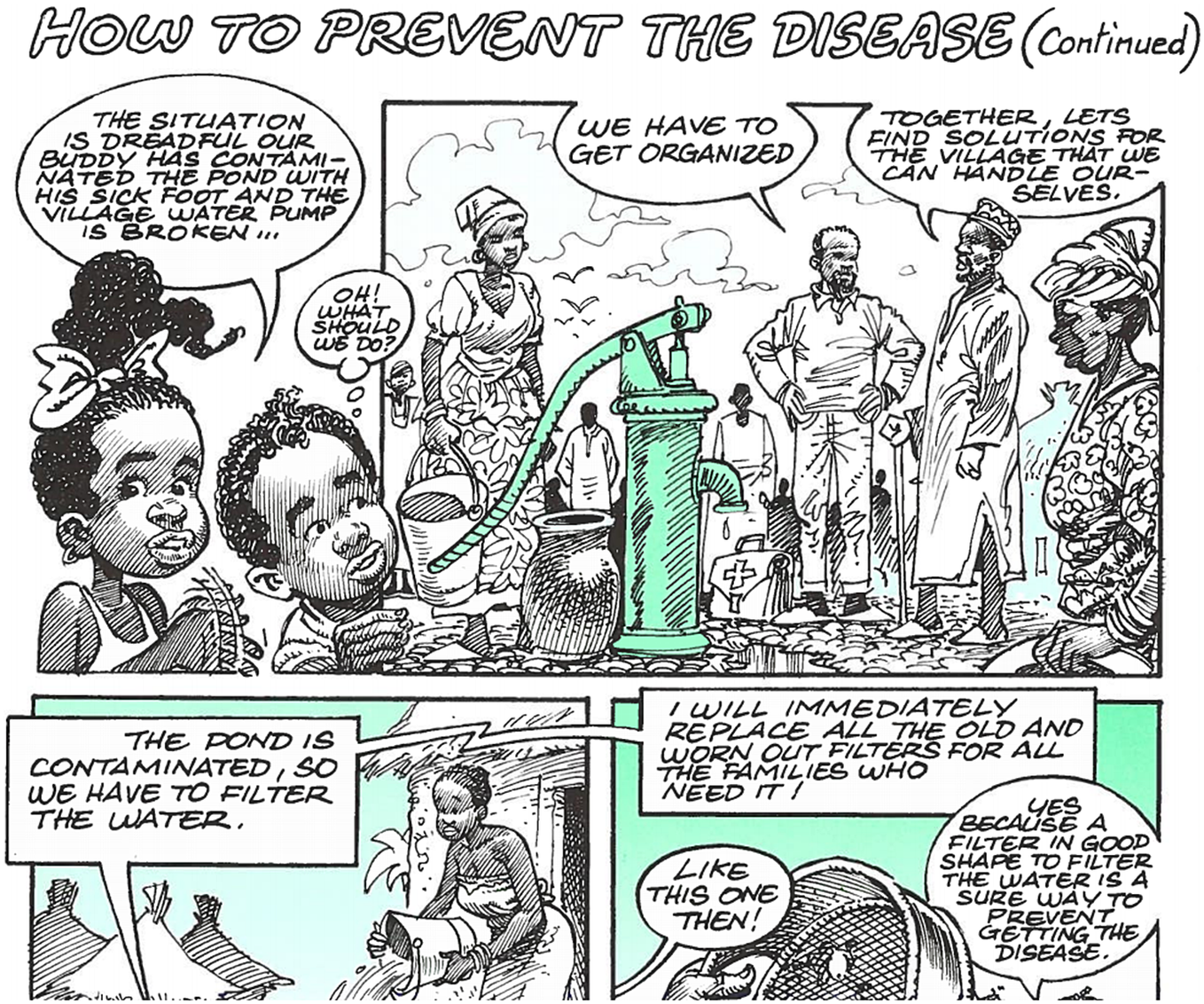
Figure 2 Community-led eradication; WHO/CDS/CEE/DRA99.2, 12.
Stepan notes that the GWEP was a rare eradication programme wherein affected populations were the primary beneficiaries, and its success relied on the participation of these affected communities.Footnote 103 This understates the innovation: through its focus on changing behaviours and community interventions, the GWEP put the solutions to dracunculiasis in the hands of those with the greatest reason to solve the problem. Not only did it embrace the ethos of refusing to accept any morbidity anywhere, it also made some of the world’s most impoverished people important, and it helped them take control of their health. The GWEP did not rely on retaining the interest of John D. Rockefeller or Cold War superpowers, but on the goodwill of those whose lives were impacted by guinea worm.
Global health does not have to be a game played by rich nations and philanthropists; the GWEP shows that ground-up programmes can be highly effective, though they might not produce charismatic figures like Fred Soper, whom historians can use as narrative scaffolding.Footnote 104
Participatory healthcare, moreover, is frequently defined, including by the Society for Participatory Medicine, in terms of the relationships between patients and health professionals such as doctors and researchers.Footnote 105 The GWEP, however, demonstrates a participatory mode of eradication requiring the involvement of entire communities – not just those currently infected, but also those previously infected and at risk of future infection. Furthermore, the community self-help described by Edungbola, the promotion of interventions such as filtration, and the importance of Village Volunteers suggests that eradication can be achieved without professional hegemony. Though led by professionals such as Edungbola, the GWEP created a mode of eradication and public health that was both de-professionalised and participatory.
The GWEP, therefore, as a product both of historical and biological constraints, offers an alternative view of eradication – not top-down, but bottom-up, driven by community and individual participation as much as institutions. Not technological, but PHC-focused and participatory, it was driven by individual and collective choice as much as WHO politics.
Part 2: twenty years of endgame challenges?
This section discusses two challenges to the GWEP that have become increasingly prominent in recent years, as Village Volunteer and health education programmes have reduced case numbers. Firstly, it addresses the challenges that conflict and movement of people, particularly refugees and nomads, have posed to the GWEP. The WHO responded to these challenges by considering guinea worm as a problem of human mobility, and by creating solutions, most notably the filter pipe drinking straw, which emphasised personal prophylaxis (individual behaviour), PHC and surveillance. Secondly, I explore a shift in the 2010s that was driven by guinea worm itself: its transformation into a zoonosis. The WHO’s response once again emphasised PHC, individual behaviour and surveillance, alongside more technological solutions such as the increased use of temephos. I argue that this is an important demonstration of human and nonhuman agency shaping human health, as well as an example of both the potential of One Health approaches and their weaknesses when applied to PHC. This section will focus entirely on Africa; by 2001, the WHO considered 13 countries dracunculiasis-endemic, in an area bounded by the Central African Republic and Uganda in the south; Sudan, Niger, Mauritania and Mali in the north; Ethiopia in the east; and a cluster of West African nations from Côte d’Ivoire to Nigeria in the west.Footnote 106 Outside of Africa, only Yemen remained in the precertification phase.Footnote 107
Here be dracunculiasis?
As village-based and local PHC programmes proved successful in reducing the incidence of dracunculiasis, in the late 1990s and early 2000s, the WHO became increasingly concerned with cross-border transmission and the problems posed by conflict in the remaining endemic zones. Village-based PHC and water schemes (where they were implemented) had worked well, but these sometimes struggled to cope with nomads, refugees or otherwise mobile people. Even among settled populations, people moved frequently between villages, and visiting friends and family in nearby villages was a frequent cause of dracunculiasis.Footnote 108 Packard has criticised models of PHC that assume a village structure which may not actually exist on the ground; the GWEP shows the WHO attempting to adapt to this reality.Footnote 109 As the GWEP entered the twenty-first century and many African countries approached eradication, mobile groups and individuals became increasingly important, as they made up a relatively higher proportion of the remaining cases. In the eyes of the WHO, this redefined guinea worm was a problem of human mobility, which resulted in an increased focus on cross-border surveillance and health education promoting safe drinking.
The main challenge for the GWEP as the twenty-first century dawned was Sudan, which not only reported the vast majority of the world’s dracunculiasis cases, but also exported guinea worm to the neighbouring non-endemic countries of Ethiopia, Kenya, Uganda and the Central African Republic within the bodies of refugees fleeing what was becoming one of the world’s longest-running conflicts, despite the 1995 Jimmy-Carter-brokered ‘Guinea Worm Ceasefire’.Footnote 110 The secession of South Sudan in 2011 provided a brief glimmer of hope for peace, but a 2021 WHO report complains, as have many reports before it, that:
Insecurity and inaccessibility due to conflicts continue to hinder eradication efforts in certain areas of the DRC, Mali, South Sudan and Sudan.Footnote 111
As eradication reaches its ‘endgame’, conflict has become an ever-more-important obstacle to eliminating the remaining focuses of guinea worm infection. Edungbola even believes that had guinea worm not been eradicated in Nigeria when it was, breakdowns of central authority across West Africa and the Sahel might have rendered it impossible.Footnote 112 Similarly, in a 2021 paper examining violence and conflict as a challenge to the GWEP, Louise Kelly-Hope and David Molyneux also identified
…the porosity of international borders, extensive migration generated by insecurity, intercountry range of many of the actors responsible for violence, and the need for these geographically vast countries to ensure that any Guinea worm case is recognised and reported…
as key obstacles to eradication.Footnote 113
The biological nature of guinea worm, with its year-long asymptomatic incubation period, further meant that the issues of conflict and cross-border transmission were intertwined. Those infected in the conflict zones that the GWEP struggled to reach could unknowingly carry guinea worm inside their bodies as they fled. Even in countries that were largely at peace, WHO reports display considerable anxiety about dracunculiasis re-entering countries which had eliminated the disease. From at least the mid-1990s WHO insisted that for a country to be certified dracunculiasis-free, it must maintain sufficient surveillance measures to enable it to find and contain cases imported from endemic neighbours.Footnote 114 Cross-border movement rendered each country reliant on the effectiveness of their neighbours’ eradication programmes. This situation is what Stepan calls ‘Soper’s Law’ – eradication in one area necessitates eradication in the areas surrounding it – but Soper relied on the assistance of stable states with institutionalised coercive power over their citizens, something which the WHO has rarely been able to exploit in Southern Sudan over the past 30 years.Footnote 115
This focus on cross-border transmission is demonstrated by the reports prepared by governments seeking dracunculiasis-free certification. Libya traced its only recent cases of dracunculiasis to a Chadian shepherd, while Saudi Arabia referred to the risks of importation from Yemen.Footnote 116 Morocco claimed that it ‘employs no foreign labour’, but a survey of its southern regions was conducted by ICT on the grounds of ‘relative proximity to endemic countries’, while Turkmenistan pointed to the fact that its only formerly endemic neighbours, Uzbekistan and Iran, no longer recorded the disease.Footnote 117 In Kenya, ICT, though convinced that the country was not endemic, insisted that the movement of nomads and refugees across the Sudanese border necessitated improved surveillance.Footnote 118 These reports show a clear concern about cross-border transmission reintroducing guinea worm to formerly endemic countries, and a recognition that tackling a disease within a country’s own borders is no guarantee of elimination unless all its neighbours are doing likewise. Morocco’s apparently closed borders were a less feasible solution for countries not surrounded by desert or sea on all sides.
In 2001, 108 imported cases of dracunculiasis were recorded in Benin (16), Burkina Faso (11), Cameroon (5), CAR (2), Cote d’Ivoire (5), Ethiopia (19), Ghana (1), Kenya (8), Mali (10), Niger (12), Senegal (1), Togo (14) and Uganda (4), although not all imported cases may have been found.Footnote 119 WHO mapping (Figure 3) of these shows the range and complexity of cross-border movement, particularly in West Africa, with infected persons travelling not only to neighbouring countries, but sometimes even crossing two borders to travel between countries such as Niger and Côte d’Ivoire.Footnote 120 Most seem to have travelled between Ghana, Togo and Benin, but there was movement along all the borders of Nigeria, Ghana and Burkina Faso, and along the southern borders of Mali and Niger (both of which border the Sahara to the north).Footnote 121
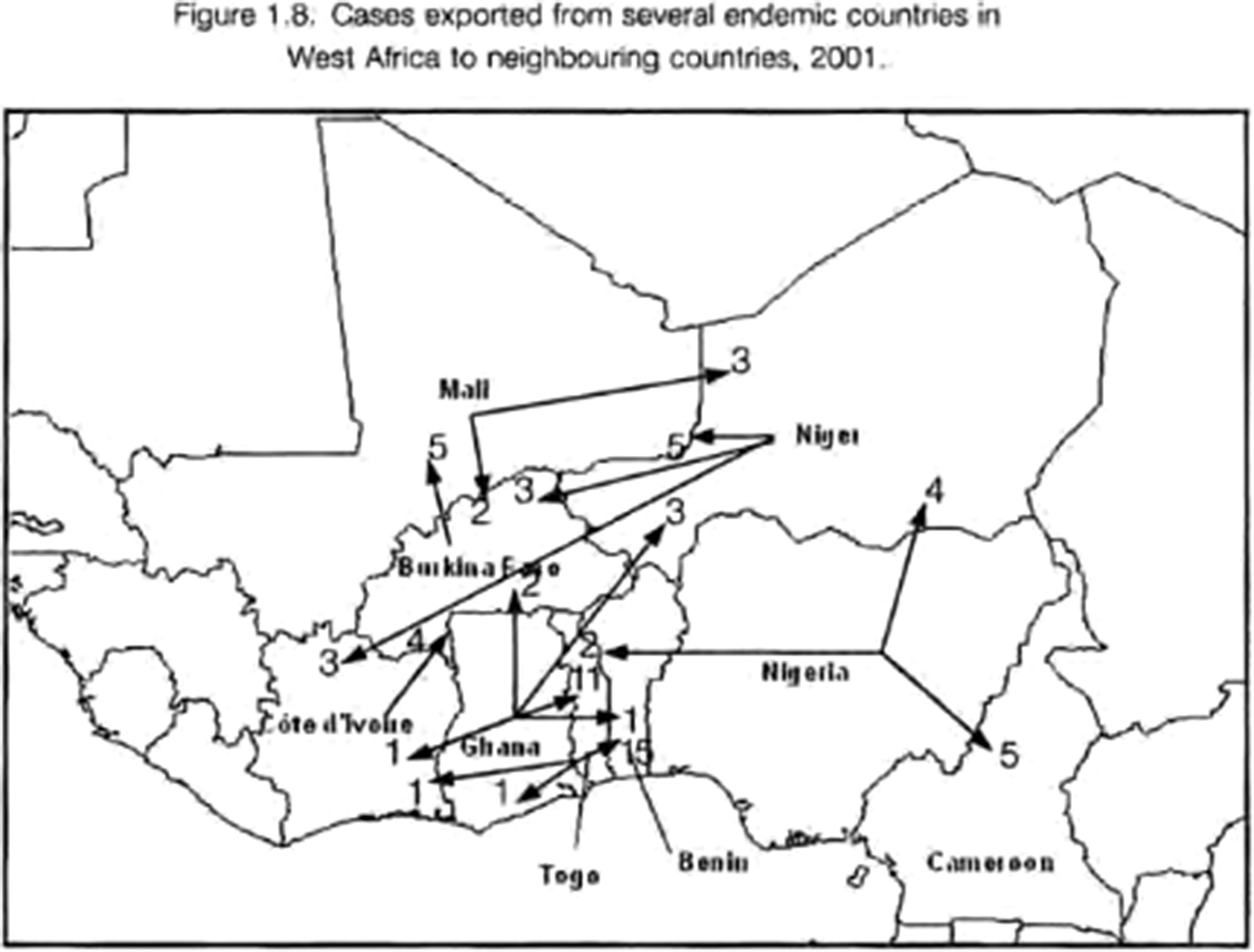
Figure 3 Cross-border movements of guinea-worm-infected people in West Africa, 2001; WHO/CDS/CPE/CEE/2002.30, 14.
The same report shows considerable flow of infected people, presumably mostly refugees, from Sudan into Ethiopia and Kenya (Figure 4). This mobility is underlined by a 2020 genomic study of guinea worm, which described an ‘East African’ population from samples from Ethiopia, South Sudan and a single Chadian sample; and a ‘West African’ population from samples from Ghana, Mali and Côte d’Ivoire, indicating significant movement of worms across borders.Footnote 122 While worms from Chad generally exhibited high levels of genetic kinship with each other, some appeared closer to South Sudanese and ‘West African’ samples, suggesting movement of worms between countries; a conclusion further substantiated by the close kinship of the Ghanaian, Malian and Ivoirian samples.Footnote 123
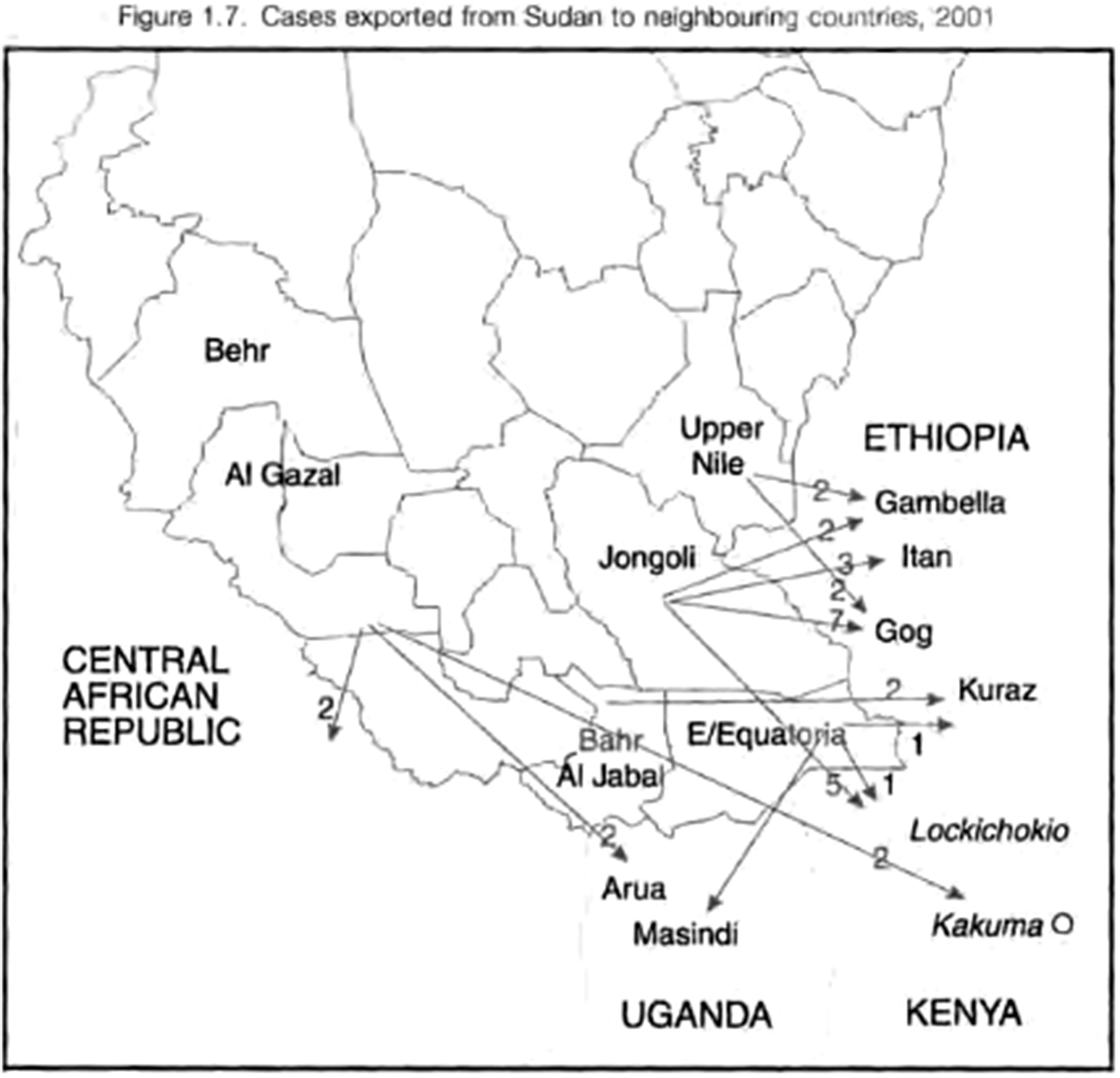
Figure 4 Cross-border movements of guinea worm victims from southern Sudan, 2001; WHO/CDS/CPE/CEE/2002.30, 13.
The mobility of nomadic populations within Africa further challenged the GWEP. Providing water at one regular habitation site is useful, but it is not possible to dig wells wherever nomadic groups travel, and surveillance systems based on villages are not necessarily applicable to mobile populations. By 2010, 95% of Mali’s recorded cases were among Touareg nomads, who were known to cross the borders of Algeria, Burkina Faso and Niger.Footnote 124 Nomadic populations in Chad have likewise been labelled a significant challenge to eradication.Footnote 125 By the late 2000s, guinea worm was seen by global health less as a development problem, and more as a problem of human mobility. However, the solutions primarily emphasised were still individual behaviour change and PHC.
One proposed solution to these problems of mobility was the adoption of filter pipe drinking straws, particularly in the highly mobile environment of Southern Sudan, as ‘the appropriate technology for people on the move, as it protects them while travelling’.Footnote 126 A filter pipe could be worn conveniently around the neck, unlike larger cloth filters, making it easier for nomadic and displaced people to protect themselves via safe drinking (Figure 5).Footnote 127 An ICT report further suggested that Kenyan nomadic groups be thought of as ‘mobile villages’ and encouraged to select their own volunteer health workers, in an attempt to stretch the village paradigm to encompass nomads.Footnote 128

Figure 5. Educational comic encouraging filter-straw use; WHO/CDS/CEE/DRA/99.2, 12.
This reliance on changing behaviours also ensured that interventions were adapted to the circumstances, with innovations such as cheap filter provision and filter pipes designed to make it easy to participate in eradication. This participatory approach, as Stepan has identified and Edungbola has charted, made eradication possible, as communities and individuals engaged with the project and were prepared to aid health workers.Footnote 129 The next section of this paper examines how the GWEP’s participatory approach responded to challenges driven not by humans, but by the agency of nonhuman animals, including dogs as well as guinea worm itself.
Dog Days
Throughout the 2000s dracunculiasis case numbers mostly continued their slow downward trajectory. But in 2010 an outbreak of guinea worm was detected in Chad, thought to have been dracunculiasis-free for a decade.Footnote 130 Even more worryingly, this occurred among people who had never travelled outside the country.Footnote 131 Chad responded by requesting WHO and Carter Centre support to search for cases and train nearly 20 000 Village Volunteers, again showcasing the GWEP’s PHC focus.Footnote 132 The subsequent investigations would cast dracunculiasis as a problem of zoonosis, rather than just behaviour and PHC, emphasisng the ability of nonhuman local actors to transform global health approaches. This section first outlines how dracunculiasis became known as a zoonosis, before discussing how the WHO has responded to this development, and finally reflecting on the potential of One Health approaches to tackling zoonotic disease and informing PHC.
In the 1980s and 1990s, WHO reports downplayed the possibility of zoonotic dracunculiasis. One 1990 report maintains:
There is no evidence that either domestic or wild animals act as reservoir hosts capable of transmitting the infection to man. Nevertheless, infection in dogs is still said to occur in regions where human dracunculiasis was formerly endemic.Footnote 133
It further argued that as most of the potential reservoir animals drink by lapping, which would drive away the turbulence-averse cyclops, their infection with guinea worm was uncommon.Footnote 134 The possibility of fish or frogs acting as either paratenic or transport hosts, trapping inside their tissues guinea worm larvae, which could then infect people who eat them, was raised and noted to be documented in related parasites of racoons and reptiles, but thought unusual.Footnote 135 By 2002 zoonotic dracunculiasis was a ‘theoretical possibility that…has not been conclusively disproved’.Footnote 136 It was noted that though cats and other animals can be infected with guinea worm experimentally, the disease had not recurred in formerly endemic areas, even where there was no piped water supply – indicating that it was not zoonotic.Footnote 137
When the investigation into the Chadian outbreak began, therefore, the assumption was that that guinea worm was mainly a human disease, and that historic cases in dogs represented ‘spillover’.Footnote 138 Humans were seen as the principal agent in infecting other animals. However, from 2011 guinea worm began to be identified in domestic dogs (these were not ‘pets’ per se, but they tended to be associated with a particular household).Footnote 139 This slow, sporadic, apparently largely epizootic outbreak, where cases could not be traced to a single water supply, formed a significant challenge to prevailing assumptions about guinea worm, as well as to the GWEP.Footnote 140
Genetic analysis was used to identify specimens to determine whether the closely related Dracunculus insignis, known to occur in American wildlife, was to blame.Footnote 141 This itself illustrates changing paradigms in guinea worm science; earlier generations of medical zoologists had relied on anatomical identification to differentiate species. But by the 2010s, this was no longer proof of species identity – the new(ish) sciences of genomics and phylogenetics were now the highest authorities in taxonomy. Genetic analysis revealed that not only were the Chadian guinea worms very different from D. insignis, the samples from dogs and humans were near-identical.Footnote 142 Zoonosis was back on the table.Footnote 143
This was taken to represent an entirely novel aetiology, a theory bolstered by the Chadians themselves, who testified that dogs had not been known to contract the worm before the present outbreak.Footnote 144 It was concluded that fish, eating infective cyclops, had indeed become paratenic hosts, which, when eaten by dogs, were able to infect the dogs, forming a complete change of transmission through a new host (Figure 6).Footnote 145 Human cases, contracted by eating undercooked fish, were therefore spillover from transmission between fish and dogs; and since surveillance of dogs has been instituted, canine infections have significantly and continuously outnumbered human infections.Footnote 146 Dogs were redefined as reservoirs and agents of infection, and dracunculiasis as a zoonosis. However, guinea worm is still influenced by a human behaviour – catching the fish eaten by companion dogs.
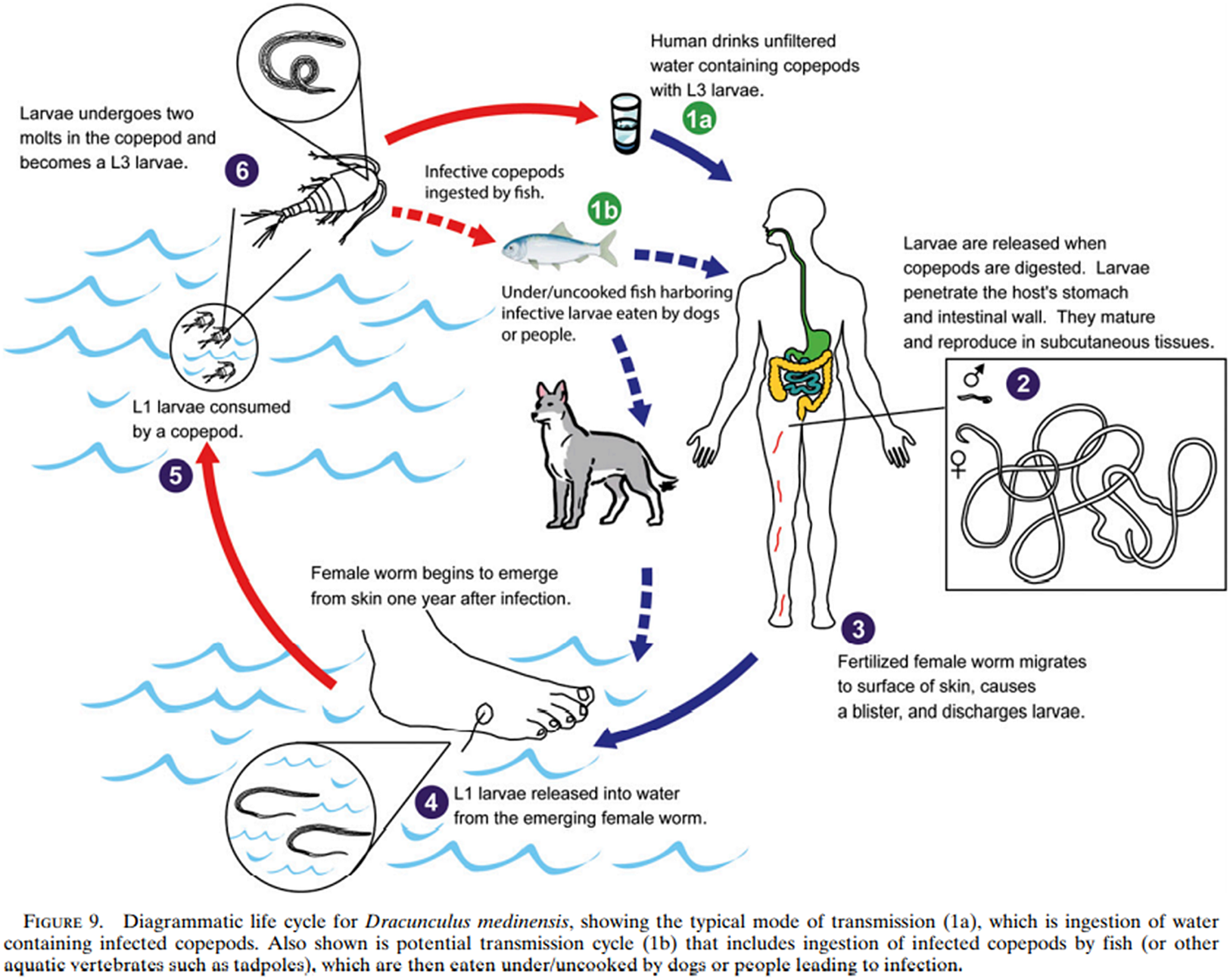
Figure 6. The new lifecycle of guinea worm, from Eberhard et al., ‘Peculiar Epidemiology’, op. cit. (note 132), 68.
For the last ten years, therefore, the WHO has been dealing with a semi-zoonotic disease, rather than the drinking-water problem it began with. And it has adapted to the changing problem: surveillance of baboons, cats and dogs has been instituted across all endemic countries, defining these animals as potential agents of infection.Footnote 147 Surveillance is still one of the WHO’s principal solutions; in these latter stages, investigations of rumours have also been prioritised, and generous cash rewards offered to help identify cases.Footnote 148 Rewards have been used since at least the 1990s towards the end of national campaigns, but the decline of dracunculiasis worldwide has made locating residual cases ever more crucial, and rewards of around US$100 (Chad) to US$350 (Ethiopia; US$17.50 for animal cases) have been instituted.Footnote 149 In several regions, surveillance of guinea worm has been integrated with surveillance of poliovirus, again demonstrating how an allegedly vertical eradication campaign (this time against poliomyelitis) can be used to leverage significant horizontal gains.Footnote 150 Health interventions have adapted to the newly zoonotic nature of dracunculiasis: while temephos has been used to kill cyclops in drinking water sources since the 1980s, its use has been increased in order to disrupt transmission within wildlife and dogs. In Chad temephos was applied to 7220 water sources in 408 villages in 2019, and 688 water sources in 82 villages in 2018, while in Ethiopia it has been regularly applied to water bodies near where infected animals and baboons have been found.Footnote 151 Containment of infected dogs has been achieved by tying them away from water sources.Footnote 152 The WHO has reacted to new developments in the biology of guinea worm, showing that the conception of a fixed microbiome identified by Frank Snowden as a fundamental part of the pre-AIDs ‘eradicationist’ paradigm has been superseded.Footnote 153 Science now recognises that the world of disease is capable of both sudden and gradual, benign and virulent, shifts. Eradication need not necessarily be ‘eradicationist’.
Here the story of guinea worm emphasises the importance of nonhuman actors in the history of disease: the worm has switched to new hosts, and fish and dogs have become important influences on human health. Guinea worm has passed from humans to dogs (zooanthroponosis) and from dogs to humans (anthropozoonosis). The epidemiology and ecology of guinea worm infections in dogs is still not fully understood, but it has become an emerging area of research since 2014, albeit one involving only around a dozen researchers.Footnote 154 This research indicates that both human and canine behaviour around fish and water can be important influences on infection, with both human seasonal fishing practices and the drinking, feeding and roaming behaviours of dogs implicated.Footnote 155
Guinea worm’s movements between humans, dogs and fish demonstrates the need for PHC and eradication programmes to consider the importance of multispecies entanglements and nonhuman agency in human health. It might also be taken as evidence of the need for a One Health approach, or at least the potential usefulness of integrating One Health with the PHC of GWEP. A One Health approach is somewhat nebulously defined by the WHO-UN Food and Agriculture Organisation-World Organisation for Animal Health Tripartite Guide to Addressing Zoonotic Diseases as
Collaboration across all sectors and disciplines responsible for health…to address zoonotic diseases and other shared health threats at the human-animal-environment interface.Footnote 156
However, little interest in dracunculiasis from One Health perspectives has materialised, despite its being a clear example of a ‘shared health threat’. That guinea worm has not yet become a totem for One Health advocates (though Cecily Goodwin et al. in 2022 claimed that guinea worm ‘highlights the importance of taking a One Health approach’), I tentatively suggest, may be illustrative of some outstanding weaknesses with One Health.Footnote 157 Firstly, One Health is still poorly defined and more often advocated than implemented. Kaylee Errecaborde et al., for instance, argued in 2019 that One Health researchers must ‘move beyond discussing the inherent need for One Health, to actually reporting on the processes, outputs and outcomes of their collaborative efforts.’Footnote 158 Similarly, the Tripartite Guide of the same year provides less of a framework for One Health collaborations and more of a framework for the creation of national frameworks.Footnote 159
Secondly, the Tripartite Guide is focused on collaborations between ‘stakeholders’ and ‘sectors’.Footnote 160 While this is crucial when considering commercial livestock, it is difficult to see what different sectors could be involved in dracunculiasis, a disease of isolated, rural and poorly-resourced areas. In the case of guinea worm, there is little need for collaboration between different ‘stakeholders’: the victims of the disease and the owners of the affected animals are even if not, as is likely, the same individuals, members of the same communities. The GWEP’s participatory PHC seems more suited to tackling this particular disease than a top-down collaboration between governments and ‘sectors’.Footnote 161
Likewise, One Health has thus far largely been concerned with livestock and wildlife, rather than companion and pet animals such as dogs.Footnote 162 This focus on animals that are commercially valuable, and therefore of concern to governments, large corporations and international conservation organisations, may hinder attention to a neglected tropical disease of isolated and resource-poor communities. One Health has not yet been adopted and reshaped by local actors as the GWEP and PHC have. Nevertheless, guinea worm may provide an opportunity for this to occur.
The complex interactions of environment, humans, dogs, fish and guinea worm illustrate both the potential usefulness of One Health and the fact that this potential has not yet been fully realised. As more zoonoses emerge, PHC may need to borrow from One Health a sensitivity to the many pathways of infection between humans and nonhuman animals, while One Health might benefit from the GWEP’s flexible bottom-up focus on the participation of the individuals and communities most at risk from zoonotic disease.
The history of guinea worm eradication demonstrates how the biological and the historical interact. The biology of guinea worm – its long incubation period, mobility and particular mode of transmission – challenged WHO and spurred the creation of a novel mode of eradication. This was influenced by the historical moment and redefined the disease and the worm in new ways. Guinea worm became a PHC problem both because of the need for prompt extraction and because of the rise of the ideology of PHC. It became a problem of promoting safe drinking both because of its waterborne nature and because of social and political factors such as the inability of governments (and the refusal of the World Bank) to provide safe water and the need of nomads and refugees to protect themselves while travelling. It became a problem of mobility because of its year-long incubation period and because of political instability and human migration.
But the new phenomena created by eradication did not stop at healthcare. Guinea worm’s biology and its ecology were profoundly altered by eradication, as it became primarily a disease of dogs, rather than humans. The WHO’s rapid adaptation to this speaks of a mutual coevolution, as both parasite and medicine creatively respond to each other. Eradication offers new windows on the interaction of biology and history, and the GWEP offers a profoundly different conception of eradication to previous eradication programmes.
The GWEP’s participatory approach allowed local actors to respond to the changing natures of guinea worm in ways suited to local circumstance, as those living at risk of dracunculiasis reshaped a global programme according to their own priorities and needs. This, moreover, has successfully driven a debilitating disease to the brink of eradication and prevented immense human suffering. The GWEP provides a valuable template for a participatory approach to public health and disease eradication, something which should not be forgotten in this age of the emerging disease.
Acknowlegements
This paper began life as the second half of my MSc dissertation in History of Science, Medicine and Technology at St. Antony’s College, University of Oxford, and is a product of the tireless help, support and advice of my supervisor, Professor Erica Charters, as much as of my own work. If this were a biology paper, she would be a co-author! I would also like to thank Serena Turton-Hughes for her insightful feedback on my drafts of this paper, as well as the reviewers for their many helpful suggestions for improvement.
Funding
This research was self-funded.
Competing interest
The authors have no competing interests to declare.








