Introduction
Legionnaires’ disease is now routinely discussed as an ‘emerging infectious disease’ (EID) and is said to be one to the earliest such diseases to be recognised. It first appeared in 1976 and its cause was identified in 1977, the same year that Ebola fever, Hantaan virus and Campylobacter jejuni arrived.Footnote 1 The designation of Legionnaires’ disease as an EID was retrospective; it was not and could not be otherwise as the category only gained currency in the early 1990s following the Institute of Medicine’s report on Emerging Infections: Microbial Threats to the United States.Footnote 2 This publication attracted strong interest in medicine and the public sphere, and helped galvanise action. The United States Centers of Disease Control led the way, founding the journal Emerging Infectious Diseases in 1995, which began with four issues per year, moved to six in 1999 and is now monthly. The subject was widely popularised, most notably in Laurie Garrett’s The Coming Plague: Newly Emerging Diseases in a World Out of Balance, published in 1994, and was taken up in the popular press, on the then new World Wide Web, and in films such as Outbreak.Footnote 3 The most prominent EID of the era was HIV/AIDS and this provided the template for the category.Footnote 4 Legionnaires’ disease was a founding member, but it is striking how inclusive the category became.Footnote 5 EIDs were typically framed as originating, on the one hand, in the adaptive power of evolving microorganisms, and on the other, in new disease ecologies created by social, economic and technological developments. In addition, there was a growing awareness of the impact of environmental changes at all levels: from global issues, such as rapid urbanisation and climate change, down to quite local factors, such as, in the case of Legionnaires’ disease, the temperature of water storage tanks.
In this article we reflect on the changing medical understanding and social profile of Legionnaires’ disease in the decade or so from its recognition to the creation of EIDs, especially its ambivalent position between public health and clinical medicine. However, we question any simple opposition between public health experts who approached Legionnaires’ disease as a new and worrying environmental threat that could be prevented, and clinicians who saw it as another cause of pneumonia that could be managed by improved diagnosis and treatment. We argue that in the British context of public spending cuts and the reform of public health, the category of ‘new’ diseases, in which Legionnaires’ disease was central, was mobilised ahead of the EID advocacy of the early 1990s, by interested groups in medicine to defend infectious diseases services.
Whilst there are popular or professional histories of many of the infectious diseases that have ‘emerged’ in the past four decades, Legionnaires’ disease has been neglected.Footnote 6 Gordon Thomas and Max Morgan-Witts’s Trauma: The Search for the Cause of Legionnaires' Disease was published in 1981 and, as the title indicates, deals largely with the early epidemiological and laboratory investigations that led Joe McDade at the Centers of Disease Control (CDC) in Atlanta to reveal Legionella pneumophila as the bacterial cause in January 1977.Footnote 7 In this article we aim to remedy the historical neglect of Legionnaires’ disease, but also to break new ground in four ways. First, we focus on the story of the disease in Britain, exploring the interplay of local contingencies and transnational flows of knowledge, practice, people and materials in the construction of a new disease entity. Second, we go beyond the microbiological focus of Thomas and Morgan-Witts, to consider the social history of the disease, especially the context of the health service changes, and the legal and political culture of Britain in the 1980s. Third, we pay detailed attention to the response of clinicians to Legionnaires’ disease, especially to changes in the management of pneumonia and other chest infections. Finally, we discuss the relationships between infectious disease experts and clinicians in hospitals and general practice, arguing that rather than those working in prevention and control being in conflict, they found common cause on many fronts, including resisting what they saw as damaging reforms and cuts to the National Health Service (NHS).
Glasgow
In September 1976, a news item appeared in The Lancet under the headline ‘Plagues and Pestilences’, reporting on a ‘mysterious illness’ which had killed many of those who had attended the American Legion Convention in Philadelphia two months earlier.Footnote 8 The report linked what it called the ‘Legion sickness’ to the closure of a hospital in Toronto due to Lassa fever, observing that: ‘We have all become a little blasé about infectious disease’. The lesson was not so much about ‘new’ infections, rather, ‘the public realise the hazards that still lurk, that poliomyelitis, diphtheria, and other infections have not been eliminated but only held at bay, that organisms are all about us.’Footnote 9
The first report of Legionnaires’ disease in Britain appeared over a year later in November 1977.Footnote 10 Curiously, the letter referred to previous cases from 1973 and June 1977, that were then being diagnosed retrospectively. The discovery had been prompted by Professor Dan Reid, then working at Ruchill Hospital, who after hearing a radio news item on the Philadelphia outbreak had reflected on similarities between what was by then known as Legionnaires’ disease, and cases of so-called ‘Benidorm pneumonia’ four years and four months earlier.Footnote 11 The first case of ‘Benidorm pneumonia’ was in a fifty-year-old Lanarkshire tourist, John Ross, who had died on the aeroplane returning from Spain on 24 July 1973.Footnote 12 At that time, Dan Reid was the local infectious diseases expert who had to cut short his holiday to respond to the developing drama. He subsequently wrote that: ‘The death of a man on an aeroplane from Spain shortly before arrival in Glasgow and the subsequent deaths soon after of two fellow passengers might be a suitable beginning for a novel by Agatha Christie or John Le Carré.’Footnote 13 By 3 August the local press had picked up the story. The front page of the Glasgow Herald announced ‘Tourists die of mystery illness’; and the next day ‘Fatal illness remains a mystery’.Footnote 14 Three days later, the paper quoted Dr Scott Wilson, the Glasgow Medical Officer of Health, as saying: ‘Our own enquiries have drawn a blank so far apart from the Pathologist findings (of pneumonia). But we may find something else eventually.’Footnote 15 That ‘something else’ turned out to be quite dramatic. It was found that of the 189 tourists staying at the Rio Park Hotel in Benidorm with Thomson Sky Tours, two-thirds had been ill, a third of them with chest illnesses, of whom nine were admitted to hospital and three died. Extensive microbiological and toxicological investigations failed to find a cause. Even the alcoholic drinks served at the hotel bar were tested for poison at Strathclyde University with negative results.Footnote 16 The investigation in 1973 had concluded that the stress on inexperienced travellers of foreign package holidays, together with unusual food, excess alcohol and sun, and personal predisposing medical factors, were to blame. The conclusion was that better advice needed to be given to intending travellers.Footnote 17
The second outbreak of ‘Benidorm pneumonia’ in July 1977 occurred when a fifty-one-year-old housewife, who had also recently returned from the Rio Park Hotel, was admitted to hospital in Glasgow with a severe pneumonia. She died within three days, in spite of being given ampicillin and gentamicin antibiotics, a common combination at that time for severe pneumonia.Footnote 18 Four months later, remembering both episodes and linking them to the news that Legionnaires’ disease had been linked to a specific bacterial infection, Reid contacted the CDC in Atlanta. He had worked there for several months in 1970 and was able to ask former colleagues to test specimens from the Scottish patients. These proved positive for Legionella pneumophila (L. pneumophila), the causative bacterium, and in October 1977 The Scotsman reported: ‘An illness that killed three Scottish holiday makers in Spain in 1973 has been traced to Legionnaires’ disease, after the Agency was informed of a case by Scottish Health Authorities after publicity about the Philadelphia outbreak.’Footnote 19
There was a second occurrence of Legionnaires’ disease in Britain in the summer of 1977, when a total of fifteen patients were admitted to Nottingham City Hospital with severe lobar pneumonia that did not respond to standard antibiotic therapies, which led doctors to suspect Legionnaires’ disease. They sent blood samples to the CDC for serological testing: two cases were definitely confirmed and another three were classified as ‘highly suggestive’.Footnote 20 This early experience of the disease and its emerging science allowed Nottingham doctors to become one of the main conduits for the importation of CDC expertise into Britain and to become advisors for subsequent British cases.Footnote 21 Alistair Macrae, a microbiologist, advised on a number of outbreaks, undertaking bacteriological examinations and his clinical colleagues set out treatment protocols.Footnote 22 The UK Public Health Service Laboratory (PHLS) also liaised with the CDC, and the PHLS Standards Laboratory produced yolk-sac grown, formalin-killed diagnostic antigens that proved to be more reliable than the plate-grown, ether (or later heat) killed ones made in Atlanta.Footnote 23 The work of the PHLS, first with Legionnaires’ disease and then with other infections such as Campylobacter, helped sustain it when its future was uncertain due to government spending cuts, though as we discuss later, its future remained in the balance for many years.
The Rio Park Hotel was back in the British news in September 1980. The Times reported, in an article headed, ‘Legionnaires’ Disease Strikes Spanish Hotel’, that yet another British tourist had died from Legionnaires’ disease after returning from a holiday there.Footnote 24 Only two days later, ten more suspected cases were reported. During August and September 1980 nearly 5,000 guests had stayed at the Rio Park Hotel and fifty-eight developed pneumonia, giving an attack rate of over one per cent. An investigation showed that an old water well had been brought back into use five days before the start of the outbreak and had fed water infected with L. pneumophila into the hotel. Those who showered and washed first thing each morning had been most at risk of contracting Legionnaires’ disease because bacteria multiplied overnight in water standing in peripheral pipe work. This has provided a useful piece of information for British travellers ever since about when not to shower in hotels.Footnote 25
The first mention of Legionnaires’ disease as a ‘new’ disease in the British press was in December 1979 in The Times on the Pittsburgh Pneumonia Agent.Footnote 26 The article reported another incidence of hospital Legionella infection, L. micdadei rather than L. pneumophila, where seven kidney transplant patients died in a year. The reporter emphasised not so much the new agent, but the vulnerability of immunosuppressed patients, drawing parallels with the fungal infections that were also being commonly reported in such patients. On 2 August 1980, the British Medical Journal published an editorial entitled, ‘Lungs and Legionnaires’ disease’, which – together with the correspondence that followed – captured the ambivalence of medical views at the time, as to whether this was just a newly recognised type of pneumonia, with some similarities to pneumococcal pneumonia, or was it a ‘new disease’ with unique laboratory and epidemiological features.Footnote 27 The number and type of such new diseases was quite long and scary:
Campylobacter enteritis, giardiasis, legionnaires’ disease, primary amoebic meningoencephalitis, and the viral haemorrhagic fevers…. In hospitals group B streptococcal infections… hospital-acquired enterovirus infections, and developments in surgical prostheses and immunosuppression have been followed by the appearance of new infections due to low-grade opportunistic pathogens.Footnote 28
There was no follow-up discussion of this report in the medical press, but the threat of ‘new diseases’ was being used in public health medicine to try and prevent the further run down of clinical, laboratory and administrative services threatened by the continued squeeze on NHS spending. In the late 1970s, the British Society for the Study of Infections (BSSI) had been established from the merger of two smaller groupings and had attracted a large non-clinician membership. The BSSI had founded the Journal of Infection in 1979, and an editorial in 1981, entitled ‘New Germs for Old?’, used Legionnaires’ disease, along with hospital infections, to demonstrate the importance of collaboration between microbiologists, epidemiologists and clinicians, and the need for continuing support of research on infectious diseases.Footnote 29
Public interest in the disease was fuelled by reports that L. pneumophila was not a new disease at all; in fact, studies on stored samples revealed that the first known case had been in the United States in 1947, and the organism had been responsible for numerous, previously mysterious, outbreaks of pneumonia as far back as 1965.Footnote 30 Dr Tony Smith, the medical correspondent of The Times reflected on this media fascination with Legionnaires’ disease in a commentary on 20 September 1978, at the time of the third Benidorm outbreak.Footnote 31 Noting that the death of a forty-six-year-old Englishman from Legionnaires’ disease was important enough for it to be the first item on the BBC News and many newspapers, he calculated that on the same day, twenty young people would have died in road accidents, 120 of lung cancer, and 250 of stroke, and that all of these deaths would have gone unreported. Nor was the media charisma of the Legionnaires’ death due to its relationship with foreign travel, as each year over a thousand travellers returned to Britain with malaria, of whom ten or more would die (all unannounced in the media). He questioned whether the name ‘Legionnaires’ disease’ might conjure up images of glamorous French garrisons in the Sahara, or an exotic, rare or obscure disease, but concluded: ‘No: For some reason Legionnaires’ disease has caught the imagination of the in-world of journalists…. What News Editors like is a story with impact – so the required elements are mystery, blood or scandal.’
The ‘mysterious’ character of Legionnaires’ disease had given it a sinister reputation. The illness was initially thought to be caused by poisoning or, what we now term ‘bioterrorism’, and the press featured it as a new, fearsome and mysterious plague: a ‘Monster Killer’ and the ‘Philly Killer’.Footnote 32 However, public anxieties remained such that the Industrial Water Society noted public fears might be a greater problem than the disease itself, observing that:
We have learned firstly that Legionnaires’ disease is a rare form of pneumonia, and secondly that it can be successfully treated…. The worst aspect of the wide publicity, is that a note of panic can sometimes be detected […] in the reactions of the man in the street to the prospect of being struck down in his prime.Footnote 33
In this context, Legionnaires’ disease also made good television. Horizon, the BBC’s flagship science programme, broadcast ‘The Hunt for the Legion Killer’ on 12 August 1982, describing it as an ‘enthralling medical detective story’.Footnote 34 The media were not alone in dramatising Legionnaires’ disease. Gordon Thomas and Max Morgan-Witts had previously written on other disaster events, such as the destruction of Guernica, the bombing of Hiroshima and the Wall Street Crash, hence their melodramatic take on the disease:
It continues to kill. Silently to claim victims, to leave death and panic in it’s wake, to defy the most sophisticated techniques to detect and destroy it. It seems to be everywhere, to steal effortlessly from continent to continent, mysteriously and easily crossing vast oceans and desert… (deaths) might be in the hundreds of thousands. One thing is clear; no human is safe from it.Footnote 35
Readers Digest ran an article in 1985, entitled, ‘Time Bomb in Our Tap Water’.Footnote 36 The thriller writer Desmond Bagley used the subject of Legionnaires’ disease in his novel Bahama Crisis (1980), playing on the link to the tourist trade. The plot involved industrial bioterrorism as a holiday resort’s water system in the Caribbean was seeded with Legionella bacteria to ruin its reputation. As Tom Mangan, the main character and owner of the hotel said: ‘Naturally Legionnaires’ disease is bad news for any hotelier. No one is likely to spend a carefree vacation in a resort hotel from which he may be carried out feet first.’Footnote 37
Professional interest was spurred by its high death rates and explosive outbreaks, the latter explained by its mode of transmission, from contaminated water through the inhalation of infected droplets into the lungs. The epidemiology of the infection also served to enhance its reputation and news value, especially as outbreaks occurred where they were least expected or wanted, for example, amongst the chronically ill in the community, people enjoying hotel holidays, and vulnerable hospital patients. In Britain, as elsewhere, Legionnaires’ disease was soon recognised as presenting, not only as epidemics with an obvious point source, but also in apparently random, unconnected ‘sporadic’ cases with no obvious common exposure to infected water mist. This situation became evident in the occurrence of Legionnaires’ disease in thirty-three people in the Dennistoun district of Glasgow in 1984.Footnote 38 What was perplexing to doctors was that many of those affected had chronic heart and lung health problems and rarely left their own upper floor tenement flats. Investigations revealed that the source was the drift from the cooling tower of a local brewery, carried by the south-westerly winds over this residential area of Glasgow. Investigations in the community showed that house-bound residents often sat by their open windows or leaned out to talk to neighbours in close-by flats on warm, sultry evenings, when the smell and drift from the brewery was often prominent on the wind.
Glasgow continued as a hot spot for Legionnaires’ disease and was in the news again in 1984 when an outbreak at Glasgow Royal Infirmary affected one surgeon and fifteen patients, of whom five died,Footnote 39 and in 1987 nearly half of the cases diagnosed in Scotland came from Greater Glasgow.Footnote 40 Reaction to one of these Glasgow outbreaks raised political questions and accusations of a cover-up. Michael Martin, Labour MP for Glasgow Springburn, called for a Public Enquiry accusing city officials of scandalously failing to disclose news of a Glasgow outbreak earlier. In defence, the Glasgow Health Board commented:
This is not a type of illness where you can advise the public to take precautions, and if we had come out with a statement a month ago it would have caused a great deal of anxiety amongst 30,000 people without being able to give them an iota of advice about what precautions should be taken… one could create a national panic if we said that all sources of water could contain Legionella.Footnote 41
However, further cases in November raised the stakes, as the Royston Hill Tenants’ Association called for the evacuation of 420 flats after social workers and home helps were withdrawn. They also consulted lawyers about taking action against the Council under the 1897 Public Health (Scotland) Act.Footnote 42
The costs of new maintenance and monitoring regimes for water services were an increasing burden for cash-strapped health authorities. In 1980, the Department of Health and Social Security issued a Health Notice describing the measures Health Authorities should take to reduce the chances of an outbreak of Legionnaires’ disease in hospitals, whilst cautioning: ‘No additional finance can be made available for these measures’.Footnote 43 Balancing the costs against the risk was a problem for individual hospitals. At the Industrial Water Society conference in 1981, one delegate commented that it would have cost £2,000 per month to raise the temperature of the hot water in their large hospital by 5°C to reduce the risk of Legionnaires’ disease. The expert panel responded sarcastically, ‘I am sure you took the right decision – I mean for £24,000 a year you could employ four nurses in the Intensive Therapy Unit and you would have saved many more lives by doing that.’Footnote 44
One way that the infection could present itself in another alarming form, apart from pneumonia, was dramatically illustrated in January 1988 after Hogmanay celebrations at Lochgoilhead, a village on the west coast of Scotland. Out of 187 guests, 170 developed flu-like symptoms. The first mention in the press was on 9 January, when the Glasgow Herald front page announced that ‘A mystery virus has struck a 100 people in the Argyll Village of Lochgoilhead,’ and three days later, ‘Investigations so far rule out meningitis and it is not thought the illness is Legionnaires’ disease.’Footnote 45 However, it later emerged that the cause was indeed a type of Legionella infection, which became known as Lochgoilhead fever. The hotel had obtained its water supply from two mountain springs, and Legionellae were cultured from the whirlpool water samples, with blood tests revealing that sufferers had antibodies to species previously unreported in Britain – Legionella micdadei.Footnote 46 However, retrospective testing showed that the illness had been seen previously, with the first cases traced back to Pontiac in Michigan in 1978 and a condition known as Pontiac fever.Footnote 47 Unlike classic Legionnaires’ disease which caused pneumonia, this presented as a self-limiting flu-like illness with an extremely high attack rate and with a very short incubation period of one or two days. There was one twist in the tale of Lochgoilhead fever which illustrated another unusual aspect of this infection. This concerned the young medical investigator from Glasgow, David Goldberg, who rushed up to the Drimsynie Hotel to initiate the investigations, and promptly succumbed to the infection himself, a fate that also affected a reporter during the Stafford outbreak.Footnote 48 David Goldberg subsequently became Professor of Public Health at Health Protection, Scotland.
Stafford
A defining event, which brought together the medical, social, legal, political and economic dimensions of Legionnaires’ disease in the 1980s, was the outbreak at the Stafford District General Hospital in the spring of 1985. This affected 175 patients and resulted in twenty-eight deaths. A narrative was presented in the official enquiry report published in the following year.Footnote 49 The first warnings were on Monday 22 April 1985 when the duty physician noted that twelve cases of pneumonia had been admitted over the previous weekend, followed by a further sixteen in the next twenty-four hours. The cause of the pneumonias was undetermined and there were soon tensions between the clinicians, who wanted to identify the infectious organism and prescribe appropriate antibiotics, and the local microbiology laboratory, which was understaffed and, the clinicians felt, was slow to react to the evolving crisis and to expedite specialist testing. Anxious for advice, some clinicians contacted regional and national experts, and the local press was quickly onto the story thereafter. A series of articles appeared, and soon the hospital besieged by reporters from the national press.Footnote 50 By the end of the week, a total of fifty patients had been admitted, six of whom had died and thirteen were critically ill, with five of these on life support. That day, two patients were found to have high antibody levels to Influenza B in their blood, and local medical experts were reporting: ‘A clear and definite assumption of a viral pneumonia’.Footnote 51 However, it was not until the following week that the regional microbiology laboratory confirmed Legionnaires’ disease, prompting clinicians to switch from penicillin and gentamicin to erythromycin; the former combination being ineffective against Legionnaires’ disease.
The local clinicians’ frustration was compounded when they heard on 3 May that Legionella bacteria had been isolated some months earlier from the hospital’s cooling tower. As one physician, Peter Daggett, put it forcefully: ‘If we had known that Legionella had been isolated at any stage from the hospital cooling tower system early in the epidemic we would have… treated every patient much more aggressively with Erythromycin and Rifampicin.’Footnote 52 On 4 May, the front page of The Times voiced criticisms regarding the delay in diagnosis, and a local MP, Bill Cash, called for an independent inquiry.Footnote 53 This was granted, when a few days later Kenneth Clark, Minister of Health announced a judicial review.Footnote 54 The enquiry was chaired by Sir John Badenoch, a distinguished physician; the other members were a QC, a professor of microbiology and two prominent engineers.Footnote 55 Proceedings began at the Gatehouse Theatre in Stafford in July, with the legal implications clear from the outset as evidence was given under oath and witnesses cross-examined. Over many months, the enquiry heard from twenty-six patients and next of kin, and sixty-four medical and non-medical professionals.
Press interest was strong. The front page headline in the Daily Mail on Saturday 4 May 1985 was ‘Hidden Killer Disease Shock’ and described a ‘killer disease sweeping the Midlands’, while on the same day the banner headline in the Daily Mirror was ‘Legionnaires’ Plaque Claims 27 Lives. Killer Disease Alert’.Footnote 56Tomorrow’s World on 9 May 1985 was devoted to Legionnaires’ disease.Footnote 57 Some coverage was meant to shock. A cartoon illustrating an article in The Observer newspaper on 11 August 1985 about the Stafford hospital outbreak showed a skeleton flying out of a hospital ward heating duct and thrusting an arm into the mouth of a terrified patient, with the clawed hand bursting out through her chest. ‘Buildings are dangerous,’ began the accompanying article, ‘sometimes they kill you’ [see Figure 1]Footnote 58

Figure 1 Cartoon illustrating an article in the Observer newspaper on Sunday 11 August 1985, page 19, about Legionnaires' disease following the Stafford Hospital outbreak. The Observer, 1985. Reproduced with permission.
The national context was one of NHS reform and cuts in the face of persistent and new challenges, not only with chronic diseases but with infections too. The latter, which focused on the fate of the PHLS, was a matter of great contention between the medical profession and the government.Footnote 59 A report proposed to save £37 million by transferring responsibility for the fifty-two regional laboratories from central government to local health authorities. A key argument in those opposing the change was the role that central co-ordination had played in the surveillance and the control in recent years of AIDS, Legionnaires’ disease and outbreaks of Salmonella, and claimed that the government was failing to appreciate the value of preventive medicine.Footnote 60 An editorial in the Journal of Infection in 1985 by Norman Grist, Professor of Infectious Diseases at the University of Glasgow and colleague of Dan Reid, argued that the demise of the PHLS had to be resisted, for the ‘old’ infections had not gone away, particularly in Third World countries and that: ‘Even “new” infections still appear’.Footnote 61 The list included pandemic acute haemorrhagic conjunctivitis, hepatitis, Legionnaires' disease, genital herpes and AIDS. However, the priority was given to AIDS on all fronts in succeeding years, beginning with the press ‘panic’ in 1985, followed by the media campaign in March 1986.
The Badenoch Inquiry’s first report, published in June 1986, concluded that the water system in the cooling tower serving the maternity unit, theatres and the out-patient department had become heavily contaminated with a virulent strain of Legionella pneumophila, which had multiplied when the system was turned off over the long Easter weekend. An infected water aerosol, created in the cooling tower, had entered the fresh air inlet duct and was inhaled by patients, visitors and staff. The danger had been compounded by a basic design and installation fault in the chiller units in the out-patient department, which allowed infected water draining from the roof cooling-tower drip-trays to be sucked into the air coolers serving the department. While presented largely as the man-made disaster, specific environmental conditions had been critical, namely unseasonably high outdoor temperatures, wind direction and humidity levels after the Easter holiday.Footnote 62 The outbreak also exposed the potential risk to employees, with 304 staff, nearly a third of those tested, showing evidence of having had a mild form of Legionella infection.Footnote 63
The Inquiry’s second report, published in December 1987, set out the importance of building and plumbing design on Legionella prevention in the NHS.Footnote 64 However, the emerging financial burden of controlling Legionnaires’ disease was already evident. The ‘Cooling Tower Task Force’, set up by the West Midlands Regional Health Authority in November 1985 following the Stafford outbreak reported that twelve of the thirteen West Midlands hospital cooling towers required either immediate or early replacement, and the DSS informed the Badenoch Committee that 270 of the 370 wet cooling towers within the NHS should be replaced with air cooled condensers.Footnote 65 These were not the only costs to affect the health service following this one outbreak. In May 1988, The Observer reported that the West Midlands Health Authority had paid out more than £500,000 in compensation to eighty-nine patients or relatives, and a further twenty claims were under investigation.Footnote 66
The NHS was not the only government body being affected by Legionnaires’ disease. Already in 1984, the civil service unions had been alarmed by the discovery of Legionella bacteria in the cooling towers of the National Engineering Laboratory in East Kilbride, tested after the Glasgow Dennistoun outbreak. This coincided with growing demands amongst civil servant unions and staff representatives that all government establishments should be tested for the presence of Legionella, and written reassurances should be given that such buildings were free of bacteria.Footnote 67 This was contrary to government policy, published in 1980 and reiterated in 1986, which recommended only regular maintenance of water systems and prompt investigation of any cluster of cases.Footnote 68 This conflict over Legionnaires’ disease came at a time of fraught relations between government and trade unions; the miners’ strike had ended in March 1985, and in the second half of the year there were disputes involving teachers, council workers, and clinical staff in medical schools.
Pressure from the Council of Civil Service Unions was renewed after the Stafford outbreak in 1985, when they asked what action the Directorate of Civil Accommodation (DCA), which looked after the civil servant offices and establishments throughout the country, was taking regarding air conditioning plants and water treatment.Footnote 69 Briefing notes prior to a meeting with the Medical Advisory Service at the Cabinet Office on 4 October 1985 illustrate DCA concerns:
Things, therefore are now relatively calm on the client and TU side fronts simply because they know we are addressing the problems… frankly with all the criticism about of PSA [Property Services Agency], even a relatively minor but public outbreak of the disease on Crown Premises would be a disaster…. One thing is for sure, we [DCA] do not dare tell clients that we are doing all we can for them whilst the MAS [Medical Advisory Service] are slightly concerned about some of the PSA’s procedures.Footnote 70
London
The ‘disaster’, coupled with huge embarrassment, occurred on 9 October 1986, when The Guardian reported the discovery of Legionella bacteria at the NHS’s headquarters at the Department of Health and Social Services (DHSS). The building was Hannibal House, a huge complex in Elephant and Castle in London, where six hundred DHSS staff worked.Footnote 71 On 8 October, K. Blackburn, the Director of Office Services had issued a Room Notice, advising any staff already on sick leave or who developed flu-like symptoms to inform their doctor, whilst reassuring staff that there was no need for the building to be evacuated.Footnote 72 However, the reassurance failed and the DHSS was soon in turmoil. Within a week, Blackburn reported that the civil service unions: ‘Seem to be seeking to maximise disruption in HQ and there is a risk that they will try to spread alarm to local offices and other areas of the Department.’Footnote 73 This followed a call from the unions for all members to walk out, and that any further meetings between the trade unions and officials should take place in another ‘safer’ building. This had been refused. As the trade union minutes report: ‘It is believed also that the OS [Official Side] Negotiating Team had recommended that the building be closed but the ‘Top of the Office’ had said no because it clashed with the Tory Party Conference and on the day involved, Norman Fowler was getting up to speak and it might embarrass him.’Footnote 74
It was not only civil servants in government buildings who were worried. On 22 October, D. Coles, the Area Works Officer of Thames Water, wrote to his regional director: ‘There is a possibility that an embarrassing situation could develop at the Princess of Wales Conservatory, Kew Gardens.’Footnote 75 Water testing at Kew had found high concentrations of Legionella bacteria in one of the sprinkler systems. Dr Chris Bartlett from the PHLS, was brought in to advise and agreed that the humidification system, fed by rain water containing debris from the roofs of the new tropical house in Kew Gardens, presented a Legionella health risk. This was likely to be a serious threat during the summer months and remedial action would be complicated by the fact that chemical treatment of the water was not possible because of its impact on the plants. Subsequently, Coles was given £50,000 to check the other Royal Household Gardens, which revealed, embarrassingly, Legionellae in the water systems at the Windsor Gardens Nursery.Footnote 76
The legal implications of Legionella outbreaks were manifold. In industry, there were potential civil claims for personal injury. Across all sectors, there were possible claims against professionals regarding their expertise and responsibilities, which might extend to criminal proceedings for putting persons at risk of harm and death by not following regulations. The Health and Safety at Work Act of 1974 had resulted in successful prosecutions; for example, the fining of the BBC and the Science Museum Trustees, as detailed later.Footnote 77 Alistair McLellan, writing in Building Technology in November 1988, estimated that civil suits within industry could run into millions of pounds and warned that: ‘The building industry needs to work fast to avoid the legal repercussions of Legionnaires’ disease epidemics…. Everyone involved in the construction of a building in which Legionnaires’ disease breaks out… will be open to criminal proceedings.’Footnote 78 The same year, J. Sykes of the Industrial Water Society suggested liabilities might be avoided:
These responsibilities are not absolute; they are qualified by ‘reasonable practicability’; quantifying the risk is particularly difficult and it may ultimately be up to the employer or person concerned to persuade a Court that precautions would not be reasonably practical.Footnote 79
The high public and press interest in Legionnaires’ disease would tip this balance in an increasingly risk-averse culture.
In April 1988, there was another high-profile outbreak in London, centred on the BBC’s Broadcasting House, with seventy-nine cases and three deaths. Only eighteen cases were BBC employees, the remainder were people who lived nearby or had passed the building. An article about the outbreak in The Sunday Times Colour Magazine in 1989 by the investigative reporter, Chris Horrie, set the tone with a headline: ‘Germ Warfare’. The article opened with the question: ‘Deadly bacteria raining down on London’s streets might seem more science fiction than fact, but Legionnaires’ disease is very real. How safe are we?’Footnote 80 The answer depended on perceptions of risk, especially in relation of health and safety at work. This event led the Commons Employment Committee to set up an inquiry into the recent experience of Legionnaires’ disease, which produced a number of recommendations on building maintenance and design, which the government was quick to criticise as being impractical or out of proportion to the risk.Footnote 81 However, the issue did not go away. The Health and Safety Executive was also flexing its regulatory muscles, with its Director General warning in February 1989 that:
There is absolutely no excuse for the recent outbreaks [of Legionnaires’ disease]. I warn those responsible for operating air conditioning plant that where Inspectors find adequate evidence that our advice has not been followed we shall prosecute.Footnote 82
During 1989, the BBC was fined £3,000 for criminal negligence, British Aerospace £4,000, and the Trustees of the Science Museum £500 with £35,000 costs.Footnote 83 The government eventually took statutory action in April 1991, introducing an ‘Approved Code of Practice’.Footnote 84 This fell short of legal requirements, but for the first time did set a standard specific to Legionnaires’ disease against which cases could be judged in court, rather than only relying on the more general Health and Safety at Work Act.Footnote 85 In Britain, therefore, Legionnaires’ disease was seen, in part, as a new hazard of work: a man-made condition where sufferers were victims of bad design and/or poor maintenance of water systems. The context was all important: the new attitudes to risk in the wake of the creation of the Health and Safety Executive (HSE) in 1974; the public service cuts of the Thatcher government; and, in consequence, the strained relations between workers, unions and employers. There was some surprise on all sides that an infectious disease was a hazard of work, and that it was one that was difficult to diagnose and did not respond to the usual antibiotics.
The Clinical Response
For clinicians, Legionnaires’ disease was first and foremost a new form of pneumonia. Once a frightening and common disease with a high mortality, pneumonia had become treatable with penicillin and other antibiotics, leading to bacteriological expertise and clinical interest in the disease waning.Footnote 86 A major textbook on lung disease published in 1973 observed that: ‘As a result of these two factors [prompt penicillin therapy and pneumococcal pneumonia becoming uncommon], lobar pneumonia, a relatively common disease prior to 1950, has almost disappeared in the last two decades.’Footnote 87 Sufferers were often treated in general practice rather than in hospital, as would have been common previously. There were three linked clinical problems with Legionnaires’ disease: (i) it presented like any other pneumonia with no reliably distinct features; (ii) laboratory diagnosis was difficult and slow; and (iii) penicillin, which was the accepted treatment for pneumonia, was ineffective against Legionella. Yet, early trials had shown that prompt therapy with other antibiotics, such as erythromycin, improved outcomes.Footnote 88 The question was when to use erythromycin? Confidence in accepted treatments was shaken by the evidence that pneumonia was not necessarily pneumococcal, and that laboratory investigation and directed antibiotics were important. This led to diagnostic and treatment practices to change for all pneumonias.
The first method for identification of the Legionella bacterium in the laboratory was developed by McDade and colleagues at the CDC in 1979.Footnote 89 It was technically demanding, lengthy and expensive. It involved injecting the ground-up lung tissue from a sufferer into guinea pigs, then taking a spleen extract from animals that became ill, then inoculating the extract into one week-old embryonated eggs which were ‘candled’ daily to detect when they died, and finally removing the infected yolk sacs for special silver staining. The profile of the disease and the increase in reported cases led to more laboratories attempting to grow Legionellae from lung secretions using special culture media, which in turn prompted doctors to develop more invasive techniques to obtain samples from the lungs of patients with pneumonia. These techniques, which included transtracheal aspiration (passing a tube through the neck into the trachea), bronchoscopy, and needle aspiration of the lung through the skin to obtain ‘lung juice’ were often unpleasant and risky for the patient, especially if performed by enthusiastic but inexperienced hospital doctors.Footnote 90 However, even operating on critically ill patients to biopsy the pneumonic lung proved to be useful.Footnote 91 Invasive tests were reported as being crucial to early diagnosis during the Glasgow Royal Infirmary Legionnaires’ disease outbreak in November 1985, which affected one surgeon and fifteen patients, of whom five died,Footnote 92 and in an outbreak in Birmingham, where early diagnosis resulted from lung biopsy, and the doctors argued that this benefit, ‘justifies the small increase in risk’.Footnote 93 Indeed, the Committee of Inquiry into the Stafford outbreak, whilst being critical of the delay in diagnosing the cause of that outbreak, was impressed by the speed with which the diagnosis was made in the Glasgow hospital outbreak. However, while resources were mobilised in local crises, the complexities of the laboratory diagnosis of Legionella meant that it was not routinely available across the country.
Physicians’ hopes for a simple, safe, rapid, sensitive and specific diagnostic test, as with other infectious diseases, were raised by developments in novel antibody and antigen tests. While much was promised and seemed possible in theory, converting prototypes into routine tests was often a lengthy process. A diagnostic Legionella urine antigen detection test was reported at the Second International Legionella Symposium at Atlanta in June 1983,Footnote 94 and in 1987, Dr Paul Edelstein predicted that ‘once (and if) a commercial kit is available… the urine antigen test will probably replace all other available rapid diagnostic tests for infections caused by Legionella.’Footnote 95 However, different companies developed different methods, markers and labels, and it was not for nearly twenty years after the discovery of Legionnaires’ disease, that a simple urine antigen test was widely available to hospitals that could reveal a result within less than half an hour. Dr Norman Moore, who developed the rapid technique into a marketable product in 1998 for Binax, noted that one major hurdle in selling the test kit in Britain was convincing hospitals that they did have cases of Legionnaires’ disease, but were just not diagnosing them.Footnote 96 However by 2006, eighty-six per cent of the diagnoses of Legionnaires’ disease in the UK were made by urinary antigen detection.Footnote 97
The early difficulties of diagnosing Legionnaires’ disease led some experts to recommend a more pragmatic approach to managing and treating all pneumonias, no longer just with a penicillin, but also with antibiotics to cover Legionnaires’ disease for most, if not all patients.Footnote 98 The cadre of Legionnaires’ disease experts in Nottingham, such as Dr Dewi Davies and Dr Andrew Miller, led the calls to rethink the management of all pneumonias.Footnote 99 General practitioners were also advised to consider Legionnaires’ disease when seeing a patient with pneumonia. In a 1983 article in The Practitioner, John Macfarlane, wrote that, ‘If recovery [from pneumonia] is slow but the patient not seriously ill, an atypical pneumonia [such as Legionnaires’ disease] is a possibility and a change to Erythromycin should be considered.’Footnote 100 A study from the Nottingham group concluded ‘early use [of erythromycin] by general practitioners, especially during the course of an epidemic and in areas where Legionnaires’ disease is known to occur, may be of advantage.’Footnote 101 As noted above, the experience of this group, and others like it from Glasgow and London, was a key resource for the development and dissemination of knowledge of Legionnaires’ disease in Britain, however, it may also have contributed to an inappropriately high profile of Legionnaires’ disease as a cause of pneumonia in medical circles, particularly when directed at doctors in localities where it was less prevalent.
Despite the attention that Legionnaires’ disease received in the press and from medical enthusiasts, the British Thoracic Society (BTS) did not investigate sporadic pneumonia until 1982, and their study of twenty-five British hospitals was not published until 1987.Footnote 102 The results showed Legionnaires’ disease to be uncommon, with only nine cases in 453 (2%) adults with community-acquired pneumonia, none of whom died, but some of whom became seriously ill. Subsequent national guidelines for the management of pneumonia also put Legionnaires’ disease into context, recommending, ‘L. pneumophila, though uncommon, can cause severe pneumonia… and is difficult to diagnose early. In seriously ill patients, antibiotics active against this organism should be started early.’Footnote 103 The Badenoch report had made similar observations a year earlier, stating that: ‘Each year in England and Wales there are about 150–200 cases [of Legionnaires’ disease] compared with an estimated annual incidence of 180,000 cases of all forms of pneumonia… although the incidence is low, the disease once contracted is serious.’Footnote 104
A further overview of the true situation in the UK was presented by the Public Health Laboratory Service Communicable Disease Surveillance Centre in March 1988, when they reported on over 1,300 cases in the previous decade in England and Wales, with a 12.5% death rate.Footnote 105 Nearly half of the cases were associated with travel, mostly involving hotels abroad, two-thirds occurred between June and September, and eight high-profile outbreaks related to hospitals in the UK, including at Kingston General Hospital in 1980 with four deaths, and the large 1985 outbreak at the Stafford District General Hospital which resulted in twenty-eight deaths.
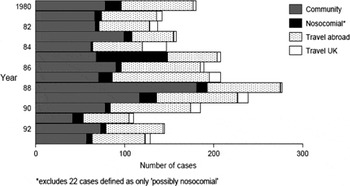
Figure 2 Reports of Legionnaires' disease in England and Wales from 1980 to 1993 showing the spectrum of sources of infection. Taken with permission from C.A. Joseph, D. Dedman, R. Birtles, J.M. Watson, C.L.R. Bartlett, Legionnaires' disease surveillance: England and Wales, 1993, Communicable Disease Report (London: Health Protection Agency, 1994), 4, R109–R111: R109. Reproduced with permission from the Health Protection Agency.
The pharmaceutical industry also responded to Legionnaires’ disease. Adverts for antibiotics to treat pneumonia in the 1960s to 1970s usually recommended a penicillin; for example, the 1973 advert for ‘Magnapen’ in the general practice magazine Pulse, ‘Pneumonia – call on the greater certainty of MAGNAPEN … Magnapen destroys virtually all bacteria met in general practice. So you can rely on Magnapen even when there are no laboratory reports to guide you.’Footnote 106 However, with the discovery and marketing of a whole new range of antibiotics, the battle between the pharmaceutical companies to persuade doctors to prescribe them increased. One strategy, used by Abbott, the makers of erythromycin, was to emphasise the importance of using an antibiotic to cover the new atypical pathogens such as Legionnaires’ disease and mycoplasma, which did not respond to penicillin. Their advertising materials were peppered with phrases such as: ‘Decisive intervention in respiratory infection’, ‘Power to attack’, ‘First time power’, ‘Powerful spectrum in pneumonia’, ‘Killing power’. When Abbott brought out their new intravenous formulation of erythromycin in December 1986, full-page colour adverts in the British Medical Journal emphasised that erythromycin had an appropriate spectrum in pneumonia.Footnote 107 The statement was supported by quotes from The Lancet and British Medical Journal, backed up by claims that the British Thoracic Society recommended immediate combined erythromycin and penicillin treatment in pneumonia. In fact, the advert used selective quotations, and perhaps unsurprisingly, the referenced articles were considerably less definite in their support for always using erythromycin.Footnote 108 In 1990, Abbott brought out a new macrolide antibiotic, clarithromycin, as a successor to erythromycin, and promoted it heavily with the slogan, ‘Chest pathogens are changing, so is the treatment.’Footnote 109 The two-page advert in the British Medical Journal featured a series of images depicting primates evolving from quadrupeds into a healthy looking human family, stating, ‘Can your antibiotic cope with changing pathogens… Klaricid (clarithromycin) is appropriate for initial therapy in community acquired respiratory tract infection’.Footnote 110
Conclusion
The story of Legionnaires’ disease in Britain demonstrates the global character of medicine in the late twentieth century. A local disease outbreak in an hotel in Philadelphia quickly becomes a news story around world, and medical agencies of all types, first nationally and then internationally, were mobilised. The relatively quick identification of its bacterial cause was an obvious success, but rather than seeing rapid progress in control, it signalled the beginning of a period when the ecology and pathology of the disease became ever more complex, and control proved elusive. The outbreaks in Glasgow, Stafford and London saw different groups within the medical profession, sanitary engineers, local and national politicians, and the news media, negotiate around different identities and approaches to control. Medically, Legionnaires’ disease was a new type of pneumonia, a public health hazard and a threat that clinicians could mobilise around to defend medical specialisms, laboratories and the NHS as a whole. For engineers, Legionella was a problem that required new technical innovations and management protocols to ensure the effective maintenance of systems, all set within the new health and safety culture and legal frameworks. Members of Parliament were drawn into controversies surrounding Legionnaires’ disease because of the ways it affected particular localities and social groups, while nationally, it registered as lobby groups used it as a symptom of cuts in public services.
Relations between clinicians, hospital laboratories and public health agencies were critical in the story of Legionnaires’ disease, at local, national and international levels. The early identification of the pathogenic bacillus at the CDC and its role, as an international reference centre, defining what were, and what were not outbreaks of the disease, were crucial in standardising the infection. This role was taken up in Britain by the PHLS, particularly by experts in the central laboratories at Colindale, which provided a much-needed reference centre for the UK. However, the diagnostic tests were complex and expensive, which meant that clinicians at the bedside, faced with seriously ill patients, tended to both treat early with broad-spectrum antibiotics, and subject patients to investigations with often-invasive procedures. Much of the early medical work on Legionnaires’ disease in Britain came from experts who worked in areas that subsequently were shown to have unusually high incidences of Legionnaires’ disease. Their interest and enthusiasm for the disease may have encouraged an overemphasis on Legionella infection as a cause of sporadic pneumonia. A national study on the frequency of the infection was not published for over a decade after Legionnaires’ disease was first described, and this found it to be a rare cause of sporadic community-acquired pneumonia, albeit with a clear tendency to cause more severe or sometimes fatal illness. This, together with the widely publicised point source outbreaks of the illness, both in the country and abroad, and some involving hospitals, served to keep the condition continuously on the medical radar. This profile was useful because infectious disease experts in both public health and hospitals, were facing reforms and demands for cost savings greater than in other areas because their diseases were seen to be in decline. Thus, they found common cause in Legionnaires’ disease and linked it with other ‘new’ infections before EIDs became the big story in the early 1990s. In an editorial in the Journal of Infectious Diseases in 1985, Norman Grist, who had worked with Dan Reid on the first Benidorm pneumonia outbreak in 1973 and had just retired as Chair of Infectious Diseases at the University of Glasgow, reflected on ‘a new awakening’ about infections.Footnote 111 He listed ‘hepatitis, Legionnaires' disease, genital herpes and AIDS’ as the diseases that had shown ‘infectious diseases have not been conquered’. His immediate target was local, the ‘threatened demise of the UK Public Health Laboratory Service’, but this was set in the larger context of ‘the health administrations of the many countries which have allowed their infectious diseases services to decline’ and the low priority given to infection ‘compared with more popular and influential medical specialties’. The experience of Legionnaires’ disease in the 1980s – medically, socially and politically – anticipated all the key features of the EID concept of the 1990s and helped enhance its reputation and influence far beyond its modest contribution as a relatively uncommon cause of pneumonia.
Acknowledgements
We are grateful to Dan Reid, Tim Harrison, Jonathon Cossar, David Goldberg and Michael Bresalier for their helpful discussions about the subject, and to Dan Reid and Tim Harrison for comments on early draft papers. Peter Stephens of IMSHealth kindly provided historical data on antibiotic use. John Macfarlane gratefully acknowledges a Wellcome Trust History of Medicine research-training grant, which made this work possible. Michael Worboys acknowledges the continuing support of the Wellcome Trust at the Wellcome Unit for the History of Medicine, University of Manchester.




