Subsolidus solubility between 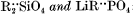 : a hydrothermal investigation
: a hydrothermal investigation
Published online by Cambridge University Press: 14 March 2018
Summary
The subsolidus phase equilibrium of the lithiophilite-tephroite system was studied in the temperature range 500 to 900° C. The solubility of tephroite in lithiophilite is limited and increases with temperature. The reaction is very sluggish and has peculiar kinetic properties. No reverse solubility was observed. Similar asymmetry was found in the LiMgPO4-forsterite system of which a preliminary study was made. Apparent absences of silicate phosphate olivine solid solutions in nature are related to our experimental findings and to geological factors.
- Type
- Research Article
- Information
- Mineralogical magazine and journal of the Mineralogical Society , Volume 35 , Issue 273 , March 1966 , pp. 742 - 755
- Copyright
- Copyright © The Mineralogical Society of Great Britain and Ireland 1966
References
- 7
- Cited by


