Crossref Citations
This article has been cited by the following publications. This list is generated based on data provided by
Crossref.
Neal, Colin
and
Stanger, Gordon
1985.
The Chemistry of Weathering.
p.
249.
Barber, D. J.
1985.
Phyllosilicates and other layer-structured materials in stony meteorites.
Clay Minerals,
Vol. 20,
Issue. 4,
p.
415.
1986.
References.
Geological Society, London, Memoirs,
Vol. 11,
Issue. 1,
p.
167.
ROTHERY, D. A.
1987.
Improved discrimination of rock units using Landsat Thematic Mapper imagery of the Oman ophiolite.
Journal of the Geological Society,
Vol. 144,
Issue. 4,
p.
587.
Craw, D.
Landis, C. A.
and
Kelsey, P. I.
1987.
Authigenic Chrysotile Formation in the Matrix of Quaternary Debris Flows, Northern Southland, New Zealand.
Clays and Clay Minerals,
Vol. 35,
Issue. 1,
p.
43.
Taylor, R. M.
Hansen, H. C. B.
Stanger, G.
and
Bender Koch, C.
1991.
On the genesis and composition of natural pyroaurite.
Clay Minerals,
Vol. 26,
Issue. 3,
p.
297.
Russell, Michael J.
Daniel, Roy M.
Hall, Allan J.
and
Sherringham, John A.
1994.
A hydrothermally precipitated catalytic iron sulphide membrane as a first step toward life.
Journal of Molecular Evolution,
Vol. 39,
Issue. 3,
p.
231.
Stanger, Gordon
and
Neal, Colin
1994.
The occurrence and chemistry of huntite from Oman.
Chemical Geology,
Vol. 112,
Issue. 3-4,
p.
247.
Kaschke, Michael
Russell, Michael J.
and
Cole, W. John
1994.
[FeS/FeS2]. A redox system for the origin of life.
Origins of life and evolution of the biosphere,
Vol. 24,
Issue. 1,
p.
43.
RUSSELL, M. J.
and
HALL, A. J.
1997.
The emergence of life from iron monosulphide bubbles at a submarine hydrothermal redox and pH front.
Journal of the Geological Society,
Vol. 154,
Issue. 3,
p.
377.
RUSSELL, MICHAEL J.
INGHAM, J. KEITH
ZEDEF, VEYSEL
MAKTAV, DERYA
SUNAR, FILIZ
HALL, ALLAN J.
and
FALLICK, ANTHONY E.
1999.
Search for signs of ancient life on Mars: expectations from hydromagnesite microbialites, Salda Lake, Turkey.
Journal of the Geological Society,
Vol. 156,
Issue. 5,
p.
869.
Kelley, Deborah S.
Karson, Jeffrey A.
Blackman, Donna K.
Früh-Green, Gretchen L.
Butterfield, David A.
Lilley, Marvin D.
Olson, Eric J.
Schrenk, Matthew O.
Roe, Kevin K.
Lebon, Geoff T.
and
Rivizzigno, Pete
2001.
An off-axis hydrothermal vent field near the Mid-Atlantic Ridge at 30° N.
Nature,
Vol. 412,
Issue. 6843,
p.
145.
Russell, M. J.
Hall, A. J.
and
Mellersh, A. R.
2003.
Natural and Laboratory-Simulated Thermal Geochemical Processes.
p.
325.
2004.
Geochemistry of hyper alkaline environments:.
Journal of Japanese Society for Extremophiles,
Vol. 3,
Issue. 1,
p.
1_60.
Palandri, James L
and
Reed, Mark H
2004.
Geochemical models of metasomatism in ultramafic systems: serpentinization, rodingitization, and sea floor carbonate chimney precipitation.
Geochimica et Cosmochimica Acta,
Vol. 68,
Issue. 5,
p.
1115.
Skinner, H. Catherine W.
2005.
The Web of Magnesium.
International Geology Review,
Vol. 47,
Issue. 11,
p.
1111.
Russell, Michael J.
Hall, Allan J.
Boyce, Adrian J.
and
Fallick, Anthony E.
2005.
100th Anniversary Special Paper: > On Hydrothermal Convection Systems and the Emergence of Life.
Economic Geology,
Vol. 100,
Issue. 3,
p.
419.
Huber, Claudia
and
Wächtershäuser, Günter
2006.
α-Hydroxy and α-Amino Acids Under Possible Hadean, Volcanic Origin-of-Life Conditions.
Science,
Vol. 314,
Issue. 5799,
p.
630.
SATO, Tsutomu
FUKUSHI, Keisuke
and
YONEDA, Tetsuro
2007.
Environmental Behavior and Management of Hazardous Inorganic Anions in Nature.
Journal of MMIJ,
Vol. 123,
Issue. 4/5,
p.
132.
Russell, Michael J.
2007.
The Alkaline Solution to the Emergence of Life: Energy, Entropy and Early Evolution.
Acta Biotheoretica,
Vol. 55,
Issue. 2,
p.
133.
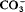 surface waters with a Ca2+-2OH− groundwater in both surface and groundwater environments. These results indicate that significant differences exist between the processes of medium- and low-temperature brucite generation.
surface waters with a Ca2+-2OH− groundwater in both surface and groundwater environments. These results indicate that significant differences exist between the processes of medium- and low-temperature brucite generation.