Article contents
The crystal structure of kiddcreekite solved using micro X-ray diffraction and the EPCryst program
Published online by Cambridge University Press: 05 July 2018
Abstract
Abundant kiddcreekite grains were identified in the Zijinshan Cu-Au epithermal deposit in Fujian Province, China. The mineral occurs as polycrystalline grains, 5–300 μm in size, associated with colusite, enargite, stannoidite, mawsonite, vinciennite, hemusite, tennantite and wolframite in a predominantly covellite ore. Based on electron microprobe analysis, the empirical formula of the kiddcreekite is Cu6.2Sn0.97W0.95S7.83, without significant Se or Te contents. The crystal structure of kiddcreekite was solved using the direct-space method (EPCryst) from laboratory micro X-ray diffraction (μXRD) data and refined by the Rietveld method. The R values of the final Rietveld refinement were Rp = 9.06%, Rwp = 8.31%, RB = 3.16 and RF = 2.17%. Kiddcreekite has a cubic structure, space group F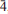 3m and lattice parameter a = 10.8178(3) Å (Z = 4, V = 1265.95(6) Å3). In the unit cell, W, Sn and Cu atoms occupy the 4a, 4c and 24f Wyckoff positions, respectively, and S atoms occupy two sets of 16e Wyckoff positions. The structure of kiddcreekite consists of stacked double MeS4 layers (giving a W–Sn–Cu tier and a Cu–vacancy tier) as in the sphalerite substructure. This study also demonstrates the possibility of using laboratory µXRD data coupled with the direct-space method to solve inorganic structures in cases where samples are too small for conventional powder and single-crystal diffraction.
3m and lattice parameter a = 10.8178(3) Å (Z = 4, V = 1265.95(6) Å3). In the unit cell, W, Sn and Cu atoms occupy the 4a, 4c and 24f Wyckoff positions, respectively, and S atoms occupy two sets of 16e Wyckoff positions. The structure of kiddcreekite consists of stacked double MeS4 layers (giving a W–Sn–Cu tier and a Cu–vacancy tier) as in the sphalerite substructure. This study also demonstrates the possibility of using laboratory µXRD data coupled with the direct-space method to solve inorganic structures in cases where samples are too small for conventional powder and single-crystal diffraction.
- Type
- Research Article
- Information
- Copyright
- Copyright © The Mineralogical Society of Great Britain and Ireland 2014
References
- 7
- Cited by


