Article contents
Ammoniovoltaite, (NH4)2Fe2+5Fe3+3Al(SO4)12(H2O)18, a new mineral from the Severo-Kambalny geothermal field, Kamchatka, Russia
Published online by Cambridge University Press: 15 May 2018
Abstract
Ammoniovoltaite, (NH4)2Fe2+5Fe3+3Al(SO4)12(H2O)18, is a new voltaite-group mineral. The mineral was discovered at the Severo-Kambalny (North-Kambalny) geothermal field, Kambalny volcanic ridge, Southern Kamchatka, Russia. Ammoniovoltaite forms at ~100°C around geothermal gas/steam vents in association with alunogen, tschermigite and pyrite. Crystals of ammoniovoltaite have euhedral habit, are up to 50 µm in size and grow on alunogen plates. Ammoniovoltaite is black with vitreous lustre, opaque, brittle and water-soluble. Neither cleavage nor parting is found, the fracture is conchoidal. The mineral is isotropic, with the refractive index n = 1.602(2) (589 nm). Infrared spectra contain an absorption band at 1433 cm–1 distinctive for the ammonium ion. The chemical composition is (iron content is given in accordance with Mössbauer data, H2O calculated from a crystal-structure refinement, wt.%): FeO 13.26, Fe2O3 11.58, MgO 2.33, ZnO 0.04, Al2O3 2.74, SO3 47.46, K2O 0.19, CaO 0.11, (NH4)2O 2.96, H2O 16.03, total 96.70. The empirical formula based on S = 12 atoms per formula unit is [(NH4)1.88K0.08Ca0.04]Σ2.00(Fe2+3.74Mg1.17Fe3+0.05Zn0.01)Σ4.97(Fe3+2.89Al0.09)Σ2.98Al1.00(SO4)12.00(H2O)18.00. The crystal structure has been refined to R1 = 0.031 and 0.030 on the basis of 1217 and 1462 unique reflections with I >2σ(I) collected at 100 K and room temperature, respectively. Ammoniovoltaite is the ammonium analogue of voltaite. The mineral is cubic, Fd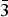 $\bar{3}$c, a = 27.250(1) Å and V = 20234(3) Å3 (at 100 K); and a = 27.322(1) Å and V = 20396(3) Å3 (at RT), with Z = 16. The strongest lines of the powder X-ray diffraction pattern [d, Å (I, %) (hkl)] are: 9.67 (74) (022), 7.90 (56) (222), 5.58 (84) (422), 3.560 (100) (731), 3.418 (100) (008) and 2.8660 (37) (931). A brief review of ammonium minerals from various volcanically active geological environments is given.
$\bar{3}$c, a = 27.250(1) Å and V = 20234(3) Å3 (at 100 K); and a = 27.322(1) Å and V = 20396(3) Å3 (at RT), with Z = 16. The strongest lines of the powder X-ray diffraction pattern [d, Å (I, %) (hkl)] are: 9.67 (74) (022), 7.90 (56) (222), 5.58 (84) (422), 3.560 (100) (731), 3.418 (100) (008) and 2.8660 (37) (931). A brief review of ammonium minerals from various volcanically active geological environments is given.
- Type
- Article
- Information
- Copyright
- Copyright © Mineralogical Society of Great Britain and Ireland 2018
Footnotes
Associate Editor: Anthony Kampf
References
- 12
- Cited by


