Article contents
Ba–Cu ordering in bariopharmacoalumite-Q2a2b2c from Cap Garonne, France
Published online by Cambridge University Press: 05 July 2018
Abstract
Bariopharmacoalumite-Q2a2b2c, Ba0.5(Cu,ZnO)0.1H0.6[Al4(OH)4(As0.9Al0.1O4)3]·5.5H2O, from the south mine of the old copper mine at Cap Garonne, France, has a 2 × 2 × 2 I-centred tetragonal superstructure of the basic pharmacosiderite-type structure. Cell parameters are a = 15.405(2) Å and c = 15.553(3) Å. The structure was determined and refined in I 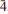 2m to R 1= 0.057 for 2697 reflections with I > 2σ(I), using synchrotron X-ray data on a twinned crystal. The origin of the superlattice cell doubling was determined to be due predominantly to the ordering of Ba atoms in half of the [0 0 1] channels, centred at (0, 0, 0) and (½, ½, 0). The other channels, centred at (½, 0, 0) and (0, ½, 0), were found to be occupied by corner-connected chains of Cu/Zn-centred square planar units.
2m to R 1= 0.057 for 2697 reflections with I > 2σ(I), using synchrotron X-ray data on a twinned crystal. The origin of the superlattice cell doubling was determined to be due predominantly to the ordering of Ba atoms in half of the [0 0 1] channels, centred at (0, 0, 0) and (½, ½, 0). The other channels, centred at (½, 0, 0) and (0, ½, 0), were found to be occupied by corner-connected chains of Cu/Zn-centred square planar units.
- Type
- Research Article
- Information
- Copyright
- Copyright © The Mineralogical Society of Great Britain and Ireland 2014
References
- 6
- Cited by


