Article contents
Belakovskiite, Na7(UO2)(SO4)4(SO3OH)(H2O)3, a new uranyl sulfate mineral from the Blue Lizard mine, San Juan County, Utah, USA
Published online by Cambridge University Press: 05 July 2018
Abstract
The new mineral belakovskiite (IMA2013-075), Na7(UO2)(SO4)4(SO3OH)(H2O)3, was found in the Blue Lizard mine, Red Canyon, White Canyon district, San Juan County, Utah, USA, where it occurs as a secondary alteration phase in association with blödite, ferrinatrite, kröhnkite, meisserite and metavoltine. Crystals of belakovskiite are very pale yellowish-green hair-like fibres up to 2 mm long and usually no more than a few mm in diameter. The fibres are elongated on [100] and slightly flattened on {021}. Crystals are transparent with a vitreous lustre. The mineral has a white streak and a probable Mohs hardness of ∼2. Fibres are flexible and elastic, with brittle failure and irregular fracture. No cleavage was observed. The mineral is readily soluble in cold H2O. The calculated density is 2.953 g cm−3. Optically, belakovskiite is biaxial (+) with α = 1.500(1), β = 1.511(1) and γ = 1.523(1) (measured in white light). The measured 2V is 87.1(6)° and the calculated 2V is 88°. The mineral is non-pleochroic. The partially determined optical orientation is X ≈ a. Electron-microprobe analysis provided Na2O 21.67, UO3 30.48, SO3 40.86, H2O 6.45 (structure), total 99.46 wt.% yielding the empirical formula Na6.83(U1.04O2)(SO4)4(S0.99O3OH)(H2O)3 based on 25 O a.p.f.u. Belakovskiite is triclinic, P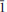 , with a = 5.4581(3), b = 11.3288(6), c = 18.4163(13) Å, α = 104.786(7)°, β = 90.092(6)°, γ = 96.767(7)°, V = 1092.76(11) Å3 and Z = 2. The eight strongest X-ray powder diffraction lines are [dobs Å(I)(hkl)]: 8.96(35)(002), 8.46(29)(011), 5.19(100)(
, with a = 5.4581(3), b = 11.3288(6), c = 18.4163(13) Å, α = 104.786(7)°, β = 90.092(6)°, γ = 96.767(7)°, V = 1092.76(11) Å3 and Z = 2. The eight strongest X-ray powder diffraction lines are [dobs Å(I)(hkl)]: 8.96(35)(002), 8.46(29)(011), 5.19(100)(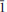 01,101,
01,101,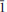 10), 4.66(58)(013,
10), 4.66(58)(013,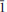 02,
02,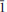
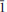 0,110), 3.568(37)(120,023,005,0
0,110), 3.568(37)(120,023,005,0 3), 3.057(59)(0
3), 3.057(59)(0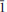 6,1
6,1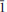 5,
5,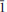 31), 2.930(27)(multiple) and 1.8320(29)(multiple). The structure, refined to R1 = 5.39% for 3163 Fo > 4σF reflections, contains [(UO2)(SO4)4(H2O)]6− polyhedral clusters connected via an extensive network of Na−O bonds and H bonds involving eight Na sites, three other H2O sites and an SO3OH (hydrosulfate) group. The 3-D framework, thus defined, is unique among known uranyl sulfate structures. The mineral is named for Dmitry Ilych Belakovskiy, a prominent Russian mineralogist and Curator of the Fersman Mineralogical Museum.
31), 2.930(27)(multiple) and 1.8320(29)(multiple). The structure, refined to R1 = 5.39% for 3163 Fo > 4σF reflections, contains [(UO2)(SO4)4(H2O)]6− polyhedral clusters connected via an extensive network of Na−O bonds and H bonds involving eight Na sites, three other H2O sites and an SO3OH (hydrosulfate) group. The 3-D framework, thus defined, is unique among known uranyl sulfate structures. The mineral is named for Dmitry Ilych Belakovskiy, a prominent Russian mineralogist and Curator of the Fersman Mineralogical Museum.
- Type
- Research Article
- Information
- Copyright
- Copyright © The Mineralogical Society of Great Britain and Ireland 2014
References
- 13
- Cited by


