Article contents
Bluebellite and mojaveite, two new minerals from the central Mojave Desert, California, USA
Published online by Cambridge University Press: 05 July 2018
Abstract
Bluebellite, Cu6[I5+O3(OH)3](OH)7Cl and mojaveite, Cu6[Te6+O4(OH)2](OH)7Cl, are new secondary copper minerals from the Mojave Desert. The type locality for bluebellite is the D shaft, Blue Bell claims, near Baker, San Bernardino County, California, while cotype localities for mojaveite are the E pit at Blue Bell claims and also the Bird Nest drift, Otto Mountain, also near Baker. The two minerals are very similar in their properties. Bluebellite is associated particularly with murdochite, but also with calcite, fluorite, hemimorphite and rarely dioptase in a highly siliceous hornfels. It forms bright bluishgreen plates or flakes up to ~20 mm 620 mm 65 mm in size that are usually curved. The streak is pale bluish green and the lustre is adamantine, but often appears dull because of surface roughness. It is non-fluorescent. Bluebellite is very soft (Mohs hardness ~1), sectile, has perfect cleavage on {001} and an irregular fracture. The calculated density based on the empirical formula is 4.746 g cm–3. Bluebellite is uniaxial (–), with mean refractive index estimated as 1.96 from the Gladstone-Dale relationship. It is pleochroic O (bluish green) >> E (nearly colourless). Electron microprobe analyses gave the empirical formula Cu5.82I0.99Al0.02Si0.12O3.11(OH)9.80Cl1.09 based on 14 (O+Cl) a.p.f.u. The Raman spectrum shows strong iodate-related bands at 680, 611 and 254 cm–1. Bluebellite is trigonal, space group R3, with the unit-cell parameters: a = 8.3017(5), c = 13.259(1) Å , V = 791.4(1) Å 3 and Z = 3. The eight strongest lines in the powder X-ray diffraction (XRD) pattern are [dobs/Å (I) (hkl)]: 4.427(99)(003), 2.664(35)(211), 2.516(100)(21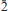 ), 2.213(9)(006), 2.103(29)(033,214), 1.899(47)(312,21
), 2.213(9)(006), 2.103(29)(033,214), 1.899(47)(312,21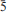 ), 1.566(48)(140,217) and 1.479(29)(045,14
), 1.566(48)(140,217) and 1.479(29)(045,14 ,324).
,324).
Mojaveite occurs at the Blue Bell claims in direct association with cerussite, chlorargyrite, chrysocolla, hemimorphite, kettnerite, perite, quartz and wulfenite, while at the Bird Nest drift, it is associated with andradite, chrysocolla, cerussite, burckhardtite, galena, goethite, khinite, mcalpineite, thorneite, timroseite, paratimroseite, quartz and wulfenite. It has also been found at the Aga mine, Otto Mountain, with cerussite, chrysocolla, khinite, perite and quartz. Mojaveite occurs as irregular aggregates of greenish-blue plates flattened on {001} and often curved, which rarely show a hexagonal outline, and also occurs as compact balls, from sky blue to medium greenish blue in colour. Aggregates and balls are up to 0.5 mm in size. The streak of mojaveite is pale greenish blue, while the lustre may be adamantine, pearly or dull, and it is non-fluorescent. The Mohs hardness is ~1. It is sectile, with perfect cleavage on {001} and an irregular fracture. The calculated density is 4.886 g cm–3, based on the empirical formulae and unit-cell dimensions. Mojaveite is uniaxial (–), with mean refractive index estimated as 1.95 from the Gladstone-Dale relationship. It is pleochroic O (greenish blue) >> E (light greenish blue). The empirical formula for mojaveite, based on 14 (O+Cl) a.p.f.u., is Cu5.92Te1.00Pb0.08Bi0.01O4(OH)8.94Cl1.06. The most intense Raman bands occur at 694, 654 (poorly resolved), 624, 611 and 254 cm–1. Mojaveite is trigonal, space group R3, with the unit-cell parameters: a = 8.316(2), c = 13.202(6) Å and V = 790.7(1) Å 3. The eight strongest lines in the powder XRD pattern are [dobs/Å (I) (hkl)]: 4.403(91)(003), 2.672(28)(211), 2.512(100)(21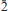 ), 2.110(27)(033,214), 1.889(34)(312,21
), 2.110(27)(033,214), 1.889(34)(312,21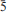 ,22
,22 ), 1.570(39)(404,140,217), 1.481(34)(045,14
), 1.570(39)(404,140,217), 1.481(34)(045,14 ,324) and 1.338(14)(422). Diffraction data could not be refined, but stoichiometries and unit-cell parameters imply that bluebellite and mojaveite are very similar in crystal structure. Structure models that satisfy bondvalence requirements are presented that are based on stackings of brucite-like Cu6MX14 layers, where M = (I or Te) and X = (O, OH and Cl). Bluebellite and mojaveite provide a rare instance of isotypy between an iodate containing I5+ with a stereoactive lone electron pair and a tellurate containing Te6+ with no lone pair.
,324) and 1.338(14)(422). Diffraction data could not be refined, but stoichiometries and unit-cell parameters imply that bluebellite and mojaveite are very similar in crystal structure. Structure models that satisfy bondvalence requirements are presented that are based on stackings of brucite-like Cu6MX14 layers, where M = (I or Te) and X = (O, OH and Cl). Bluebellite and mojaveite provide a rare instance of isotypy between an iodate containing I5+ with a stereoactive lone electron pair and a tellurate containing Te6+ with no lone pair.
Keywords
- Type
- Research Article
- Information
- Copyright
- Copyright © The Mineralogical Society of Great Britain and Ireland 2014
References
- 21
- Cited by


