No CrossRef data available.
Article contents
Brandãoite, [BeAl2(PO4)2(OH)2(H2O)4](H2O), a new Be–Al phosphate mineral from the João Firmino mine, Pomarolli farm region, Divino das Laranjeiras County, Minas Gerais State, Brazil: description and crystal structure
Published online by Cambridge University Press: 29 June 2018
Abstract
Brandãoite, [BeAl2(PO4)2(OH)2(H2O)4](H2O), is a new Be–Al phosphate mineral from the João Firmino mine, Pomarolli farm region, Divino das Laranjeiras County, Minas Gerais State, Brazil, where it occurs in an albite pocket with other secondary phosphates, including beryllonite, atencioite and zanazziite, in a granitic pegmatite. It occurs as colourless acicular crystals <10 µm wide and <100 µm long that form compact radiating spherical aggregates up to 1.0–1.5 mm across. It is colourless and transparent in single crystals and white in aggregates, has a white streak and a vitreous lustre, is brittle and has conchoidal fracture. Mohs hardness is 6, and the calculated density is 2.353 g/cm3. Brandãoite is biaxial (+), α = 1.544, β = 1.552 and γ = 1.568, all ± 0.002; 2Vobs = 69.7(10)° and 2Vcalc = 71.2°. No pleochroism was observed. Brandãoite is triclinic, space group P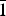 $\bar{1}$, a = 6.100(4), b = 8.616(4), c = 10.261(5) Å, α = 93.191(11), β = 95.120(11), γ = 96.863(11)°, V = 532.1(8) Å3 and Z = 2. Chemical analysis of a 4 µm wide needle-shaped crystal by electron microprobe and secondary-ion mass spectrometry gave P2O5 = 28.42, Al2O3 = 20.15, BeO = 4.85, H2O = 21.47 and sum = 74.89 wt.%. The empirical formula, normalised on the basis of 15 anions pfu with (OH) = 2 and (H2O) = 5 apfu (from the crystal structure) is Be0.98Al1.99P2.02H12O15. The crystal structure was solved by direct methods and refined to an R1 index of 7.0%. There are two P sites occupied by P5+, two Al sites occupied by octahedrally coordinated Al3+, and one Be site occupied by tetrahedrally coordinated Be2+. There are fifteen anions, two of which are (OH) groups and five of which are (H2O) groups. The simplified ideal formula is thus [BeAl2(PO4)2(OH)2(H2O)4](H2O) with Z = 2. Beryllium and P tetrahedra share corners to form a four-membered ring. Aluminium octahedra share a common vertex to form an [Al2φ11] dimer, and these dimers are cross-linked by P tetrahedra to form a complex slab of polyhedra parallel to (001). These slabs are cross-linked by BeO2(OH)(H2O) tetrahedra, with interstitial (H2O) groups in channels that extend along [100].
$\bar{1}$, a = 6.100(4), b = 8.616(4), c = 10.261(5) Å, α = 93.191(11), β = 95.120(11), γ = 96.863(11)°, V = 532.1(8) Å3 and Z = 2. Chemical analysis of a 4 µm wide needle-shaped crystal by electron microprobe and secondary-ion mass spectrometry gave P2O5 = 28.42, Al2O3 = 20.15, BeO = 4.85, H2O = 21.47 and sum = 74.89 wt.%. The empirical formula, normalised on the basis of 15 anions pfu with (OH) = 2 and (H2O) = 5 apfu (from the crystal structure) is Be0.98Al1.99P2.02H12O15. The crystal structure was solved by direct methods and refined to an R1 index of 7.0%. There are two P sites occupied by P5+, two Al sites occupied by octahedrally coordinated Al3+, and one Be site occupied by tetrahedrally coordinated Be2+. There are fifteen anions, two of which are (OH) groups and five of which are (H2O) groups. The simplified ideal formula is thus [BeAl2(PO4)2(OH)2(H2O)4](H2O) with Z = 2. Beryllium and P tetrahedra share corners to form a four-membered ring. Aluminium octahedra share a common vertex to form an [Al2φ11] dimer, and these dimers are cross-linked by P tetrahedra to form a complex slab of polyhedra parallel to (001). These slabs are cross-linked by BeO2(OH)(H2O) tetrahedra, with interstitial (H2O) groups in channels that extend along [100].
Keywords
- Type
- Article
- Information
- Copyright
- Copyright © Mineralogical Society of Great Britain and Ireland 2018
Footnotes
Deceased 2014
Associate Editor: Peter Leverett


