Published online by Cambridge University Press: 08 May 2019
The new minerals camanchacaite, NaCaMg2[AsO4]2[AsO3(OH)2], chinchorroite, Na2Mg5(As2O7)2(AsO3OH)2(H2O)10, espadaite, Na4Ca3Mg2[AsO3(OH)]2[AsO2(OH)2]10(H2O)6·H2O, magnesiofluckite, CaMg(AsO3OH)2(H2O)2, picaite, NaCa[AsO3OH][AsO2(OH)2] and ríosecoite, Ca2Mg(AsO3OH)3(H2O)2, were discovered on two closely related specimens collected from the Torrecillas mine, Iquique Province, Chile. These minerals occur as secondary phases on massive quartz–hematite also in association with anhydrite, gypsum, halite and talmessite. Camanchacaite is monoclinic, C2/c, a = 12.470(9), b = 12.554(9), c = 6.848(9) Å, β = 113.75(2)°, V = 981.3(16) Å3 and Z = 4. It has a protonated alluaudite-type structure. Chinchorroite is triclinic, P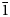 $\bar{1}$, a = 8.7777(2), b = 8.8570(3), c = 9.7981(7) Å, α = 91.097(6), β = 110.544(8), γ = 103.167(7)°, V = 690.43(7) Å3 and Z = 1. The structure contains abbreviated chains of five edge-sharing Mg octahedra that are linked by pyroarsenate and hydrogen-arsenate groups. Espadaite is orthorhombic, Ccca, a = 12.3649(10), b = 22.181(2), c = 18.3292(13) Å, V = 5027.1(7) Å3 and Z = 4. The structure is based on heteropolyhedral sheets of formula {Ca3Mg2[AsO3(OH)]2[AsO2(OH)2]10}4− that contain large voids; NaO6 polyhedra occupy the interlayer region. Magnesiofluckite is triclinic, P
$\bar{1}$, a = 8.7777(2), b = 8.8570(3), c = 9.7981(7) Å, α = 91.097(6), β = 110.544(8), γ = 103.167(7)°, V = 690.43(7) Å3 and Z = 1. The structure contains abbreviated chains of five edge-sharing Mg octahedra that are linked by pyroarsenate and hydrogen-arsenate groups. Espadaite is orthorhombic, Ccca, a = 12.3649(10), b = 22.181(2), c = 18.3292(13) Å, V = 5027.1(7) Å3 and Z = 4. The structure is based on heteropolyhedral sheets of formula {Ca3Mg2[AsO3(OH)]2[AsO2(OH)2]10}4− that contain large voids; NaO6 polyhedra occupy the interlayer region. Magnesiofluckite is triclinic, P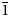 $\bar{1}$, a = 8.4143(6), b = 7.5321(5), c = 6.8917(4) Å, α = 82.477(6), β = 97.682(6), γ = 95.379(6)°, V = 427.84(5) Å3 and Z = 2. It is isostructural with fluckite. Picaite is monoclinic, P21/c, a = 7.2474(4), b = 14.6547(7), c = 7.2624(5) Å, β = 99.520(7)°, V = 760.70(8) Å3 and Z = 4. The structure contains chains of edge-sharing Na− and Ca octahedra with bridging AsO3(OH) and AsO2(OH)2 tetrahedra. Ríosecoite is triclinic, P
$\bar{1}$, a = 8.4143(6), b = 7.5321(5), c = 6.8917(4) Å, α = 82.477(6), β = 97.682(6), γ = 95.379(6)°, V = 427.84(5) Å3 and Z = 2. It is isostructural with fluckite. Picaite is monoclinic, P21/c, a = 7.2474(4), b = 14.6547(7), c = 7.2624(5) Å, β = 99.520(7)°, V = 760.70(8) Å3 and Z = 4. The structure contains chains of edge-sharing Na− and Ca octahedra with bridging AsO3(OH) and AsO2(OH)2 tetrahedra. Ríosecoite is triclinic, P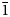 $\bar{1}$, a = 6.8110(9), b = 7.3156(12), c = 11.7773(17) Å, α = 83.466(6), β = 84.394(6), γ = 79.779(6)°, V = 571.95(15) Å3 and Z = 2. The structure contains tetramers of edge-sharing CaO7 and CaO8 polyhedra linked by MgO6 octahedra and bridging AsO3(OH) groups to form chains.
$\bar{1}$, a = 6.8110(9), b = 7.3156(12), c = 11.7773(17) Å, α = 83.466(6), β = 84.394(6), γ = 79.779(6)°, V = 571.95(15) Å3 and Z = 2. The structure contains tetramers of edge-sharing CaO7 and CaO8 polyhedra linked by MgO6 octahedra and bridging AsO3(OH) groups to form chains.
Associate Editor: Irina O Galuskina