Published online by Cambridge University Press: 02 January 2018
The new mineral centennialite (IMA 2013-110), CaCu3(OH)6Cl2·nH2O, was identified from three cotype specimens originating from the Centennial Mine, Houghton County, Michigan, USA, where it occurs as a secondary product, after acid water action upon supergene Cu mineralization in association with, and essentially indivisible from, other copper-containing minerals such as calumetite and atacamite family minerals. It forms as pale to azure blue encrustations, often taking a botryoidal form. Centennialite is trigonal, 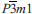 , a = 6.6606(9) Å, c = 5.8004(8) Å, V= 222.85(6) Å3, Z= 1. The strongest powder X-ray diffraction lines are dobs/Å [I%] (hkl), 5.799 [100] (001), 2.583 [75] (201), 2.886 [51] (111), 1.665 [20] (220), 1.605 [17] (023), 1.600 [15] (221), 1.444 [11] (222). The X-ray refined structure forms a kagome net of planar coordinated CuO4 units with Jahn-Teller coordinated Cl apices to form octahedra that edge-share to in-plane adjacent and flattened CaO6 octahedra, which are centred about the lattice origin. All oxygen sites are protonated and shared between one Ca-octahedron and one CuO4 planar unit. Three protonated sites are linked, by hydrogen-bonding to Cl sites, which sit on the triad axis. Each lattice has one Cl above and one below the Ca-Cu polyhedral plane. Consequently, the layers are stacked, along ⟨001⟩, with two Cl sites between layers. In addition to this kapellasite-like topology, an extra c/2 site is identified as being variably water-hosting and extends the coordination of the Ca-site to 8-fold, akin to the body-diagonal Pb-Cu sheet in murdochite. Centennialite conforms to the description of the ‘Unidentified Cu-Ca-Cl Mineral’ noted in Heinrich's Mineralogy of Michigan and is almost certainly identical to the supposed hexagonal basic calcium-copper hydroxychloride monohydrate of Erdös et al. (1981). We comment upon relationships between calumetite and centennialite and propose a substructure model for a synthetic calumetite-like phase that is related directly to centennialite.
, a = 6.6606(9) Å, c = 5.8004(8) Å, V= 222.85(6) Å3, Z= 1. The strongest powder X-ray diffraction lines are dobs/Å [I%] (hkl), 5.799 [100] (001), 2.583 [75] (201), 2.886 [51] (111), 1.665 [20] (220), 1.605 [17] (023), 1.600 [15] (221), 1.444 [11] (222). The X-ray refined structure forms a kagome net of planar coordinated CuO4 units with Jahn-Teller coordinated Cl apices to form octahedra that edge-share to in-plane adjacent and flattened CaO6 octahedra, which are centred about the lattice origin. All oxygen sites are protonated and shared between one Ca-octahedron and one CuO4 planar unit. Three protonated sites are linked, by hydrogen-bonding to Cl sites, which sit on the triad axis. Each lattice has one Cl above and one below the Ca-Cu polyhedral plane. Consequently, the layers are stacked, along ⟨001⟩, with two Cl sites between layers. In addition to this kapellasite-like topology, an extra c/2 site is identified as being variably water-hosting and extends the coordination of the Ca-site to 8-fold, akin to the body-diagonal Pb-Cu sheet in murdochite. Centennialite conforms to the description of the ‘Unidentified Cu-Ca-Cl Mineral’ noted in Heinrich's Mineralogy of Michigan and is almost certainly identical to the supposed hexagonal basic calcium-copper hydroxychloride monohydrate of Erdös et al. (1981). We comment upon relationships between calumetite and centennialite and propose a substructure model for a synthetic calumetite-like phase that is related directly to centennialite.
Powder XRD file number at the International Centre for Diffraction Data, http://www.icdd.com/