Crossref Citations
This article has been cited by the following publications. This list is generated based on data provided by
Crossref.
Kampf, A. R.
and
Mills, S. J.
2010.
Lead hydrogen citrate monohydrate, Pb(C6H6O7)·H2O, formation during specimen cleaning: a cautionary mineralogical tale.
Mineralogical Magazine,
Vol. 74,
Issue. 4,
p.
683.
Mills, Stuart J.
Kampf, Anthony R.
Raudsepp, Mati
and
Birch, William D.
2010.
The crystal structure of waylandite from Wheal Remfry, Cornwall, United Kingdom.
Mineralogy and Petrology,
Vol. 100,
Issue. 3-4,
p.
249.
Grey, I. E.
Shanks, F. L.
Wilson, N. C.
Mumme, W. G.
and
Birch, W. D.
2011.
Carbon incorporation in plumbogummite-group minerals.
Mineralogical Magazine,
Vol. 75,
Issue. 1,
p.
145.
Maubec, N.
Lahfid, A.
Lerouge, C.
Wille, G.
and
Michel, K.
2012.
Characterization of alunite supergroup minerals by Raman spectroscopy.
Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy,
Vol. 96,
Issue. ,
p.
925.
Cooper, M. A.
and
Hawthorne, F. C.
2012.
Refinement of the crystal structure of zoned philipsbornite–hidalgoite from the Tsumeb mine, Namibia, and hydrogen bonding in the D2+G3+3(T5+O4)(TO3OH)(OH)6 alunite structures.
Mineralogical Magazine,
Vol. 76,
Issue. 4,
p.
839.
Frost, Ray L.
Palmer, Sara J.
Xi, Yunfei
Čejka, Jiří
Sejkora, Jiří
and
Plášil, Jakub
2013.
Raman spectroscopic study of the hydroxy-phosphate mineral plumbogummite PbAl3(PO4)2(OH,H2O)6.
Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy,
Vol. 103,
Issue. ,
p.
431.
Christy, Andrew G.
and
Mills, Stuart J.
2013.
Effect of lone-pair stereoactivity on polyhedral volume and structural flexibility: application to TeIVO6octahedra.
Acta Crystallographica Section B Structural Science Crystal Engineering and Materials,
Vol. 69,
Issue. 5,
p.
446.
Sahlström, Fredrik
Arribas, Antonio
Dirks, Paul
Corral, Isaac
and
Chang, Zhaoshan
2017.
Mineralogical Distribution of Germanium, Gallium and Indium at the Mt Carlton High-Sulfidation Epithermal Deposit, NE Australia, and Comparison with Similar Deposits Worldwide.
Minerals,
Vol. 7,
Issue. 11,
p.
213.
Kang, Duan
Wu, Xiang
Yuan, Guan
Huang, Sheng-Xuan
Niu, Jing-Jing
Gao, Jing
and
Qin, Shan
2018.
High-pressure synchrotron x-ray diffraction and Raman spectroscopic study of plumbogummite.
Chinese Physics B,
Vol. 27,
Issue. 1,
p.
017402.
Batista, Araína Hulmann
Melo, Vander Freitas
Rate, Andrew W.
Gilkes, Robert J.
Saunders, Martin
and
Dodd, Aaron
2018.
Scanning and transmission analytical electron microscopy (STEM-EDX) can identify structural forms of lead by mapping of clay crystals.
Geoderma,
Vol. 310,
Issue. ,
p.
191.
Siidra, Oleg
Nekrasova, Diana
Depmeier, Wulf
Chukanov, Nikita
Zaitsev, Anatoly
and
Turner, Rick
2018.
Hydrocerussite-related minerals and materials: structural principles, chemical variations and infrared spectroscopy.
Acta Crystallographica Section B Structural Science, Crystal Engineering and Materials,
Vol. 74,
Issue. 2,
p.
182.
Siidra, Oleg I.
Nazarchuk, Evgeny V.
Agakhanov, Atali A.
and
Polekhovsky, Yury S.
2019.
Aleutite [Cu5O2](AsO4)(VO4)·(Cu0.5□0.5)Cl, a new complex salt-inclusion mineral with Cu2+substructure derived from a Kagome-net.
Mineralogical Magazine,
Vol. 83,
Issue. 6,
p.
847.
Pauliš, Petr
Vrtiška, Luboš
Dolníček, Zdeněk
Sejkora, Jiří
Bureš, Bohuslav
Malíková, Radana
and
Pour, Ondřej
2022.
Supergenní mineralizace hydrotermálního Ag-Pb-Zn ložiska Hříva u Louňovic pod Blaníkem (Česká republika).
Bulletin Mineralogie Petrologie,
Vol. 30,
Issue. 1,
p.
124.
Liang, Weilin
Ren, Tao
Guan, Shenjin
Yi, Yougen
Zhang, Dengmin
Yang, Tao
Luo, Xingwan
Huang, Jianguo
Zhang, Qi
and
Bao, Guangping
2023.
Gallium distribution in the Dulong Sn polymetallic deposit, SW China.
Ore Geology Reviews,
Vol. 163,
Issue. ,
p.
105821.
Kampf, Anthony R.
Désor, Joy
and
Ma, Chi
2024.
Karlseifertite, Pb(Ga2Ge)(AsO4)2(OH)6, a new dussertite-group mineral, from Tsumeb, Namibia.
European Journal of Mineralogy,
Vol. 36,
Issue. 5,
p.
873.
Vrtiška, Luboš
Zikeš, Jiří
Vrtišková, Radana
Dolníček, Zdeněk
and
Sejkora, Jiří
2024.
Studium přechodných členů řady kintoreit-segnitit-beudantit a doprovodných fosfátů z lokality Vlastkovec u Slavonic (Česká republika).
Bulletin Mineralogie Petrologie,
Vol. 32,
Issue. 2,
p.
133.
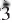 m, with the cell parameters a = 7.0752(19) Å, c = 16.818(4) Å and V = 729.1(3) Å3. The crystal structure has been refined to R1 = 2.05%. Ga-rich plumbogummite has an alunite-type structure comprised of a rhombohedral stacking of (001) composite layers of corner-shared (Al,Ga)O6 octahedra and PO4 tetrahedra, with Pb atoms occupying icosahedrally coordinated sites between the layers. The Pb and H positions are discussed. Ga-rich plumbogummite is nonpleochroic, uniaxial (+), with indices of refraction, ε = 1.742(3) and ω = 1.722(3), determined in white light. The five strongest powder-diffraction lines [d in Å, (I/I°), (hkl)] are: 2.995, (100), (113); 5.766, (95), (101); 2.236, (43), (107, 122); 3.539, (38), (110); 1.919 (32), (303, 033).
m, with the cell parameters a = 7.0752(19) Å, c = 16.818(4) Å and V = 729.1(3) Å3. The crystal structure has been refined to R1 = 2.05%. Ga-rich plumbogummite has an alunite-type structure comprised of a rhombohedral stacking of (001) composite layers of corner-shared (Al,Ga)O6 octahedra and PO4 tetrahedra, with Pb atoms occupying icosahedrally coordinated sites between the layers. The Pb and H positions are discussed. Ga-rich plumbogummite is nonpleochroic, uniaxial (+), with indices of refraction, ε = 1.742(3) and ω = 1.722(3), determined in white light. The five strongest powder-diffraction lines [d in Å, (I/I°), (hkl)] are: 2.995, (100), (113); 5.766, (95), (101); 2.236, (43), (107, 122); 3.539, (38), (110); 1.919 (32), (303, 033).

