Article contents
The crystal-structure determination and redefinition of eztlite, Pb22+ Fe33+(Te4+O3)3(SO4)O2Cl
Published online by Cambridge University Press: 15 May 2018
Abstract
The crystal structure of eztlite has been determined using single-crystal synchrotron X-ray diffraction and supported using electron microprobe analysis and powder diffraction. Eztlite, a secondary tellurium mineral from the Moctezuma mine, Mexico, is monoclinic, space group Cm, with a = 11.466(2) Å, b = 19.775(4) Å, c = 10.497(2) Å, β = 102.62(3)° and V = 2322.6(9) Å3. The chemical formula of eztlite has been revised to 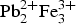 ${\rm Pb}_{\rm 2}^{2 +} {\rm Fe}_3^{3 +} $(Te4+O3)3(SO4)O2Cl from that stated previously as
${\rm Pb}_{\rm 2}^{2 +} {\rm Fe}_3^{3 +} $(Te4+O3)3(SO4)O2Cl from that stated previously as 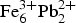 ${\rm Fe}_6^{3 +} {\rm Pb}_{\rm 2}^{2 +} $(Te4+O3)3(Te6+O6)(OH)10·nH2O. This change has been accepted by the Commission on New Minerals, Nomenclature and Classification of the International Mineralogical Association, Proposal 18-A. Eztlite was reported originally to be a mixed-valence Te oxysalt; however the crystal structure, bond-valence analysis and charge balance considerations clearly show that all Te is tetravalent. Eztlite contains a unique combination of elements and is only the second Te oxysalt to contain both sulfate and chloride. The crystal structure of eztlite contains mitridatite-like layers, with a repeating triangular nonameric [
${\rm Fe}_6^{3 +} {\rm Pb}_{\rm 2}^{2 +} $(Te4+O3)3(Te6+O6)(OH)10·nH2O. This change has been accepted by the Commission on New Minerals, Nomenclature and Classification of the International Mineralogical Association, Proposal 18-A. Eztlite was reported originally to be a mixed-valence Te oxysalt; however the crystal structure, bond-valence analysis and charge balance considerations clearly show that all Te is tetravalent. Eztlite contains a unique combination of elements and is only the second Te oxysalt to contain both sulfate and chloride. The crystal structure of eztlite contains mitridatite-like layers, with a repeating triangular nonameric [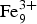 ${\rm Fe}_9^{3 +} $O36]45– arrangement formed by nine edge-sharing Fe3+O6 octahedra, decorated by four trigonal pyramidal Te4+O3 groups, compared to PO4 or AsO4 tetrahedra in mitridatite-type minerals. In eztlite, all four tellurite groups associated with one nonamer are orientated with the lone pair of the Te atoms pointing in the same direction, whereas in mitridatite the central tetrahedron is orientated in the opposite direction to the others. In mitridatite-type structures, interlayer connections are formed exclusively via Ca2+ and water molecules, whereas the eztlite interlayer contains Pb2+, sulfate tetrahedra and Cl–. Interlayer connectivity in eztlite is achieved primarily by connections via the long bonds of Pbφ8 and Pbφ9 groups to sulfate tetrahedra and to Cl–. Secondary connectivity is via Te–O and Te–Cl bonds.
${\rm Fe}_9^{3 +} $O36]45– arrangement formed by nine edge-sharing Fe3+O6 octahedra, decorated by four trigonal pyramidal Te4+O3 groups, compared to PO4 or AsO4 tetrahedra in mitridatite-type minerals. In eztlite, all four tellurite groups associated with one nonamer are orientated with the lone pair of the Te atoms pointing in the same direction, whereas in mitridatite the central tetrahedron is orientated in the opposite direction to the others. In mitridatite-type structures, interlayer connections are formed exclusively via Ca2+ and water molecules, whereas the eztlite interlayer contains Pb2+, sulfate tetrahedra and Cl–. Interlayer connectivity in eztlite is achieved primarily by connections via the long bonds of Pbφ8 and Pbφ9 groups to sulfate tetrahedra and to Cl–. Secondary connectivity is via Te–O and Te–Cl bonds.
Information
- Type
- Article
- Information
- Copyright
- Copyright © Mineralogical Society of Great Britain and Ireland 2019
Footnotes
Associate Editor: Ferdinando Bosi
Present address: School of Earth, Atmosphere and Environment, Monash University, Clayton 3800, Victoria, Australia.
References
- 5
- Cited by

