Article contents
Excess free energies of mixing of Sr,Ba-bearing binary feldspar solid solutions (experimental data)
Published online by Cambridge University Press: 05 July 2018
Abstract
The distribution of potassium and strontium, potassium and barium between feldspars and aqueous chloride solutions was studied at 700°C, 2 kbar and 800°C, 2 kbar respectively. The distribution of Sr and Ba between coexisting feldspar and chloride solutions is not ideal; Sr and Ba are enriched in feldspar relative to solution. In order to estimate the phase state of the chloride solutions, special experiments were conducted using the synthetic fluid-inclusion method in the systems KCl-BaCl2-H2O and KCl-SrCl2-H2O at 700–800°C, 2 kbar. It was shown that (0.5M KCl+0.5M SrCl2) aqueous solution is homogeneous at 800°C, 2 kbar. The 0.25–0.5M SrCl2 solutions are homogeneous at 700°C 2, kbar. The (0.5M KCl+0.5M BaCl2) solution is homogeneous at 700°C, 2 kbar and heterogeneous at 800°C, 2 kbar. Margules parameters for describing the excess energy of mixing of the (K,Sr)- and (K,Ba)-feldspar solid solutions were calculated. The integrated values of excess energy of mixing (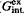 ) were estimated for the different binary feldspar solid solutions, and a correlation of
) were estimated for the different binary feldspar solid solutions, and a correlation of 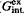 with the crystallochemical parameter ρ = (RA-RB)/D(Me-O) was observed. Feldspar solid solutions can be subdivided into three groups based on the relationship between
with the crystallochemical parameter ρ = (RA-RB)/D(Me-O) was observed. Feldspar solid solutions can be subdivided into three groups based on the relationship between 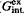 , p and the stability of the solid solution.
, p and the stability of the solid solution.
- Type
- Mineralogy
- Information
- Copyright
- Copyright © The Mineralogical Society of Great Britain and Ireland 1995
References
- 1
- Cited by


