Article contents
Ferri-fluoro-katophorite from Bear Lake diggings, Bancroft area, Ontario, Canada: a new species of amphibole, ideally Na(NaCa)(Mg4Fe3+)(Si7Al)O22F2
Published online by Cambridge University Press: 02 July 2018
Abstract
Ferri-fluoro-katophorite is the second species characterised involving the rootname katophorite in the sodium–calcium subgroup of the amphibole supergroup. The mineral and its name were approved by the International Mineralogical Association Commission on New Minerals, Nomenclature and Classification, IMA2015-096. It was found in the Bear Lake diggings, Bancroft area, Ontario, Canada, where coarse euhedral crystals of amphibole, phlogopite, sanidine solid-solution (now coarsely exsolved to microcline perthite), titanite, augite, zircon and fluorapatite crystallised from a low-viscosity silicocarbonatitic magma of crustal origin. Greenish grey prismatic crystals of ferri-fluoro-katophorite generally protrude from the walls into a body of coarsely crystalline calcite, but they also occur away from the walls, completely enclosed by calcite. The empirical formula derived from electron microprobe analysis and single-crystal structure refinement is: A(Na0.55K0.32)Σ0.87B(Na0.79Ca1.18Mn2+0.03)Σ2.00C(Mg3.29Mn2+0.02Fe2+1.19Fe3+0.31Al0.09Ti4+0.08Li0.02)Σ5.00T(Si7.39Al0.61)Σ8.00O22W[F1.23 (OH)0.77]Σ2.00. Ferri-fluoro-katophorite is biaxial (–), with α = 1.640(2), β = 1.652(2), γ = 1.658(2), 2Vmeas. = 68.9(2)° and 2Vcalc.. = 70.1°. The unit-cell parameters are a = 9.887(3), b = 18.023(9), c = 5.292(2) Å, β = 104.66(3)°, V = 912.3(6) Å3, Z = 2 and space group C2/m. The strongest ten lines in the powder X-ray pattern [d values (in Å) I (hkl)] are: 2.708, 100, (151); 2.388, 74, (131); 3.139, 72, (310); 8.449, 69, (110); 2.540, 65, (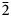 $\bar{2}$02); 2.591, 53, (061); 2.739, 47, (
$\bar{2}$02); 2.591, 53, (061); 2.739, 47, (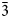 $\bar{3}$31); 2.165, 45, (261); 3.279, 44, (
$\bar{3}$31); 2.165, 45, (261); 3.279, 44, (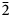 $\bar{2}$40); 2.341, 43, (
$\bar{2}$40); 2.341, 43, (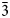 $\bar{3}$51).
$\bar{3}$51).
Keywords
- Type
- Article
- Information
- Copyright
- Copyright © Mineralogical Society of Great Britain and Ireland 2018
Footnotes
Associate Editor: Ian Graham
References
- 3
- Cited by


