Article contents
Feynmanite, a new sodium uranyl sulfate mineral from Red Canyon, San Juan County, Utah, USA
Published online by Cambridge University Press: 28 May 2018
Abstract
The new mineral feynmanite, Na(UO2)(SO4)(OH)·3.5H2O, was found in both the Blue Lizard and Markey mines, San Juan County, Utah, USA, where it occurs as a secondary phase on pyrite-rich asphaltum in association with chinleite-(Y), gypsum, goethite, natrojarosite, natrozippeite, plášilite, shumwayite (Blue Lizard) and wetherillite (Markey). The mineral is pale greenish yellow with a white streak and fluoresces bright greenish white under a 405 nm laser. Crystals are transparent with a vitreous lustre. It is brittle, with a Mohs hardness of ~2, irregular fracture and one perfect cleavage on {010}. The calculated density is 3.324 g cm–3. Crystals are thin needles or blades, flattened on {010} and elongate on [100], exhibiting the forms {010}, {001}, {101} and {10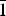 $\bar{1}$}, and are up to ~0.1 mm in length. Feynmanite is optically biaxial (–), with α = 1.534(2), β = 1.561(2) and γ = 1.571(2) (white light); 2Vmeas. = 62(2)°; no dispersion; and optical orientation: X = b, Y ≈ a,Z ≈ c. It is weakly pleochroic: X = colourless, Y = very pale green yellow and Z = pale green yellow (X < Y < Z). Electron microprobe analyses (WDS mode) provided (Na0.84Fe0.01)(U1.01O2)(S1.01O4)(OH)·3.5H2O. The five strongest powder X-ray diffraction lines are [dobs Å(I)(hkl)]: 8.37(100)(010), 6.37(33)(
$\bar{1}$}, and are up to ~0.1 mm in length. Feynmanite is optically biaxial (–), with α = 1.534(2), β = 1.561(2) and γ = 1.571(2) (white light); 2Vmeas. = 62(2)°; no dispersion; and optical orientation: X = b, Y ≈ a,Z ≈ c. It is weakly pleochroic: X = colourless, Y = very pale green yellow and Z = pale green yellow (X < Y < Z). Electron microprobe analyses (WDS mode) provided (Na0.84Fe0.01)(U1.01O2)(S1.01O4)(OH)·3.5H2O. The five strongest powder X-ray diffraction lines are [dobs Å(I)(hkl)]: 8.37(100)(010), 6.37(33)(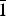 $\bar{1}$01,101), 5.07(27)(
$\bar{1}$01,101), 5.07(27)(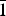 $\bar{1}$11,111), 4.053(46)(004,021) and 3.578(34)(120). Feynmanite is monoclinic, has space group P2/n, a = 6.927(3), b = 8.355(4), c = 16.210(7) Å, β = 90.543(4)°, V = 938.1(7) Å3 and Z = 4. The structure of feynmanite (R1 = 0.0371 for 1879 Io > 2σI) contains edge-sharing pairs of pentagonal bipyramids that are linked by sharing corners with SO4 groups, yielding a [(UO2)2(SO4)2(OH)2]2– sheet based on the phosphuranylite anion topology. The sheet is topologically identical to those in deliensite, johannite and plášilite. The dehydration of feynmanite to plášilite results in interlayer collapse involving geometric reconfiguration of the sheets and the ordering of Na.
$\bar{1}$11,111), 4.053(46)(004,021) and 3.578(34)(120). Feynmanite is monoclinic, has space group P2/n, a = 6.927(3), b = 8.355(4), c = 16.210(7) Å, β = 90.543(4)°, V = 938.1(7) Å3 and Z = 4. The structure of feynmanite (R1 = 0.0371 for 1879 Io > 2σI) contains edge-sharing pairs of pentagonal bipyramids that are linked by sharing corners with SO4 groups, yielding a [(UO2)2(SO4)2(OH)2]2– sheet based on the phosphuranylite anion topology. The sheet is topologically identical to those in deliensite, johannite and plášilite. The dehydration of feynmanite to plášilite results in interlayer collapse involving geometric reconfiguration of the sheets and the ordering of Na.
Keywords
- Type
- Article
- Information
- Copyright
- Copyright © Mineralogical Society of Great Britain and Ireland 2018
Footnotes
Associate Editor: Mark Welch
Current address: School of Mechanical and Materials Engineering, Washington State University, Pullman, WA 99164, USA
References
- 7
- Cited by


