Published online by Cambridge University Press: 28 February 2018
The crystal structure of vigrishinite, ideally NaZnTi4(Si2O7)2O3(OH)(H2O)4, a murmanite-group mineral of the seidozerite supergroup from the type locality, Mt. Malyi Punkaruaiv, Lovozero alkaline massif, Kola Peninsula, Russia, was refined in space group C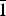 $\bar 1$, a = 10.530(2), b = 13.833(3), c = 11.659(2) Å, α = 94.34(3), β = 98.30(3), γ = 89.80(3)°, V = 1675.5(2.1) Å3 and R1 = 12.52%. Based on electron-microprobe analysis, the empirical formula calculated on 22 (O + F), with two constraints derived from structure refinement, OH + F = 1.96 pfu and H2O = 3.44 pfu, is: (Na0.67Zn0.21Ca0.05□1.07)Σ2 (Zn0.86□1.14)Σ2(Zn0.14□0.36)Σ0.5(Ti2.60Nb0.62Mn0.30
$\bar 1$, a = 10.530(2), b = 13.833(3), c = 11.659(2) Å, α = 94.34(3), β = 98.30(3), γ = 89.80(3)°, V = 1675.5(2.1) Å3 and R1 = 12.52%. Based on electron-microprobe analysis, the empirical formula calculated on 22 (O + F), with two constraints derived from structure refinement, OH + F = 1.96 pfu and H2O = 3.44 pfu, is: (Na0.67Zn0.21Ca0.05□1.07)Σ2 (Zn0.86□1.14)Σ2(Zn0.14□0.36)Σ0.5(Ti2.60Nb0.62Mn0.30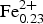 ${\rm Fe}_{{\rm 0}{\rm. 23}}^{{\rm 2 +}} $Mg0.10Zr0.06Zn0.05Al0.03Ta0.01)Σ4(Si4.02O14) [O2.60(OH)1.21F0.19]Σ4[(H2O)3.44(OH)0.56]Σ4{Zn0.24P0.03K0.03Ba0.02} with Z = 4. It seems unlikely that constituents in the {} belong to vigrishinite itself. The crystal structure of vigrishinite is an array of TS blocks (Titanium Silicate) connected via hydrogen bonds. The TS block consists of HOH sheets (H = heteropolyhedral and O = octahedral) parallel to (001). In the O sheet, the Ti-dominant MO(1,2) sites, Na-dominant MO(3) and □-dominant MO(4) sites give ideally Na□Ti2 pfu. In the H sheet, the Ti-dominant MH(1,2) sites, Zn-dominant AP(1) and vacant AP(2) sites give ideally Zn□Ti2 pfu. The MH and AP(1) polyhedra and Si2O7 groups constitute the H sheet. The ideal structural formula of vigrishinite of the form
${\rm Fe}_{{\rm 0}{\rm. 23}}^{{\rm 2 +}} $Mg0.10Zr0.06Zn0.05Al0.03Ta0.01)Σ4(Si4.02O14) [O2.60(OH)1.21F0.19]Σ4[(H2O)3.44(OH)0.56]Σ4{Zn0.24P0.03K0.03Ba0.02} with Z = 4. It seems unlikely that constituents in the {} belong to vigrishinite itself. The crystal structure of vigrishinite is an array of TS blocks (Titanium Silicate) connected via hydrogen bonds. The TS block consists of HOH sheets (H = heteropolyhedral and O = octahedral) parallel to (001). In the O sheet, the Ti-dominant MO(1,2) sites, Na-dominant MO(3) and □-dominant MO(4) sites give ideally Na□Ti2 pfu. In the H sheet, the Ti-dominant MH(1,2) sites, Zn-dominant AP(1) and vacant AP(2) sites give ideally Zn□Ti2 pfu. The MH and AP(1) polyhedra and Si2O7 groups constitute the H sheet. The ideal structural formula of vigrishinite of the form 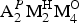 ${\rm A}_{\rm 2}^{P} {\rm M}_{\rm 2}^{\rm H} {\rm M}_{\rm 4}^{\rm O} $(Si2O7)2(
${\rm A}_{\rm 2}^{P} {\rm M}_{\rm 2}^{\rm H} {\rm M}_{\rm 4}^{\rm O} $(Si2O7)2(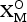 ${\rm X}_{\rm M}^{\rm O} $)2(
${\rm X}_{\rm M}^{\rm O} $)2(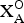 ${\rm X}_{\rm A}^{\rm O} $)2(
${\rm X}_{\rm A}^{\rm O} $)2(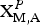 ${\rm X}_{{\rm M,A}}^{P} $)4 is Zn□Ti2Na□Ti2(Si2O7)2O2O(OH)(H2O)4. Vigrishinite is a Zn-bearing, Na-poor and OH-rich analogue of murmanite, ideally Na2Ti2Na2Ti2(Si2O7)2O2O2(H2O)4. Murmanite and vigrishinite are related by the following substitution: H(
${\rm X}_{{\rm M,A}}^{P} $)4 is Zn□Ti2Na□Ti2(Si2O7)2O2O(OH)(H2O)4. Vigrishinite is a Zn-bearing, Na-poor and OH-rich analogue of murmanite, ideally Na2Ti2Na2Ti2(Si2O7)2O2O2(H2O)4. Murmanite and vigrishinite are related by the following substitution: H(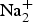 ${\rm Na}_{\rm 2}^{\rm +} $)mur + O(Na+)mur + O(O2–)mur ↔ H(Zn2+)vig + H(□)vig + O(□)vig + O[(OH)–]vig. The doubling of the t1 and t2 translations of vigrishinite compared to those of murmanite is due to the order of Zn and □ in the H sheet and Na and □ in the O sheet of vigrishinite.
${\rm Na}_{\rm 2}^{\rm +} $)mur + O(Na+)mur + O(O2–)mur ↔ H(Zn2+)vig + H(□)vig + O(□)vig + O[(OH)–]vig. The doubling of the t1 and t2 translations of vigrishinite compared to those of murmanite is due to the order of Zn and □ in the H sheet and Na and □ in the O sheet of vigrishinite.
Associate Editor: Peter Leverett