Published online by Cambridge University Press: 04 November 2019
Exhalative mineral assemblages from fumaroles of Tolbachik volcano are very rich in anhydrous sulfate minerals of alkali and transition metals. Koryakite, ideally NaKMg2Al2(SO4)6, was found in the Yadovitaya fumarole of the Second scoria cone of the North Breach of the Great Tolbachik Fissure Eruption (1975–1976), Tolbachik volcano, Kamchatka Peninsula, Russia. Koryakite occurs as a product of fumarolic activity and closely associates with euchlorine and langbeinite. Koryakite is trigonal, R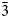 $\bar{3}$, a = 8.1124(11), c = 22.704(7) Å and V = 1294.0(5) Å3. The chemical composition determined by electron-microprobe analysis is (wt.%): Na2O 4.27, K2O 5.85, ZnO 0.31, СaO 0.31, CuO 0.76, MgO 10.15, Al2O3 11.47, Fe2O3 2.73, SO3 64.33 and SiO2 0.13, total 100.31. The empirical formula calculated on the basis of 24 O apfu is Na1.03K0.93(Mg1.89Cu0.07Ca0.04Zn0.03)Σ2.03(Al1.68Fe3+0.26)Σ1.94(S6.02Si0.02)Σ6.04O24. No natural or synthetic chemical analogues of koryakite are known to date. The topology of the [M2+2M3+2(SO4)6]2– heteropolyhedral framework in koryakite is very similar to the one in millosevichite, Al2(SO4)3 and mikasaite, Fe3+2(SO4)3. Replacement of part of the trivalent cations in the [M3+2(SO4)3]0 framework by divalent cations gives the framework a negative charge for koryakite and allows the incorporation of the alkali species in the channels. This structural mechanism is reminiscent of the concept of stuffed derivative structures. Koryakite is also structurally related to synthetic NaMgFe3+(SO4)3 and to the broader family of NASICON-related phases.
$\bar{3}$, a = 8.1124(11), c = 22.704(7) Å and V = 1294.0(5) Å3. The chemical composition determined by electron-microprobe analysis is (wt.%): Na2O 4.27, K2O 5.85, ZnO 0.31, СaO 0.31, CuO 0.76, MgO 10.15, Al2O3 11.47, Fe2O3 2.73, SO3 64.33 and SiO2 0.13, total 100.31. The empirical formula calculated on the basis of 24 O apfu is Na1.03K0.93(Mg1.89Cu0.07Ca0.04Zn0.03)Σ2.03(Al1.68Fe3+0.26)Σ1.94(S6.02Si0.02)Σ6.04O24. No natural or synthetic chemical analogues of koryakite are known to date. The topology of the [M2+2M3+2(SO4)6]2– heteropolyhedral framework in koryakite is very similar to the one in millosevichite, Al2(SO4)3 and mikasaite, Fe3+2(SO4)3. Replacement of part of the trivalent cations in the [M3+2(SO4)3]0 framework by divalent cations gives the framework a negative charge for koryakite and allows the incorporation of the alkali species in the channels. This structural mechanism is reminiscent of the concept of stuffed derivative structures. Koryakite is also structurally related to synthetic NaMgFe3+(SO4)3 and to the broader family of NASICON-related phases.
Associate Editor: Koichi Momma