Article contents
Manganoblödite, Na2Mn(SO4)2·4H2O, and cobaltoblödite, Na2Co(SO4)2·4H2O: two new members of the blödite group from the Blue Lizard mine, San Juan County, Utah, USA
Published online by Cambridge University Press: 05 July 2018
Abstract
Two new minerals – manganoblödite (IMA2012–029), ideally Na2Mn(SO4)2·4H2O, and cobaltoblödite (IMA2012–059), ideally Na2Co(SO4)2·4H2O, the Mn-dominant and Co-dominant analogues of blödite, respectively, were found at the Blue Lizard mine, San Juan County, Utah, USA. They are closely associated with blödite (Mn-Co-Ni-bearing), chalcanthite, gypsum, sideronatrite, johannite, quartz and feldspar. Both new minerals occur as aggregates of anhedral grains up to 60 μm (manganoblödite) and 200 μm (cobaltoblödite) forming thin crusts covering areas up to 2 × 2 cm on the surface of other sulfates. Both new species often occur as intimate intergrowths with each other and also with Mn-Co-Ni-bearing blödite. Manganoblödite and cobaltoblödite are transparent, colourless in single grains and reddish-pink in aggregates and crusts, with a white streak and vitreous lustre. Their Mohs' hardness is ∼2½. They are brittle, have uneven fracture and no obvious parting or cleavage. The measured and calculated densities are Dmeas = 2.25(2) g cm−3 and Dcalc = 2.338 g cm−3 for manganoblödite and Dmeas = 2.29(2) g cm−3 and Dcalc = 2.347 g cm−3 for cobaltoblödite. Optically both species are biaxial negative. The mean refractive indices are α = 1.493(2), β = 1.498(2) and γ = 1.501(2) for manganoblödite and α = 1.498(2), β = 1.503(2) and γ = 1.505(2) for cobaltoblödite. The chemical composition of manganoblödite (wt.%, electron-microprobe data) is: Na2O 16.94, MgO 3.29, MnO 8.80, CoO 2.96, NiO 1.34, SO3 45.39, H2O (calc.) 20.14, total 98.86. The empirical formula, calculated on the basis of 12 O a.p.f.u., is: Na1.96(Mn0.44Mg0.29Co0.14Ni0.06)Σ0.93S2.03O8·4H2O. The chemical composition of cobaltoblödite (wt.%, electron-microprobe data) is: Na2O 17.00, MgO 3.42, MnO 3.38, CoO 7.52, NiO 2.53, SO3 45.41, H2O (calc.) 20.20, total 99.46. The empirical formula, calculated on the basis of 12 O a.p.f.u., is: Na1.96(Co0.36Mg0.30Mn0.17Ni0.12)Σ 0.95S2.02O8·4H2O. Both minerals are monoclinic, space group P21/a, with a = 11.137(2), b = 8.279(1), c = 5.5381(9) Å, β = 100.42(1)°, V = 502.20(14) Å3 and Z = 2 (manganoblödite); and a = 11.147(1), b = 8.268(1), C = 5.5396(7) Å, β = 100.517(11)°, V = 501.97(10) Å3 and Z = 2 (cobaltoblödite). The strongest diffractions from X-ray powder pattern [listed as (d,Å(I)(hkl)] are for manganoblödite: 4.556(70)(210, 011); 4.266(45)(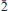 01); 3.791(26)(
01); 3.791(26)(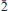 11); 3.338(21)(310); 3.291(100)(220, 021), 3.256(67)(211,
11); 3.338(21)(310); 3.291(100)(220, 021), 3.256(67)(211, 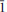 21), 2.968(22)(
21), 2.968(22)(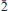 21), 2.647(24)(
21), 2.647(24)(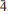 01); for cobaltoblödite: 4.551(80)(210, 011); 4.269(50)(
01); for cobaltoblödite: 4.551(80)(210, 011); 4.269(50)(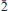 01); 3.795(18)(
01); 3.795(18)(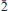 11); 3.339(43)(310); 3.29(100)(220, 021), 3.258(58)(
11); 3.339(43)(310); 3.29(100)(220, 021), 3.258(58)(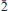 11,
11, 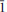 21), 2.644(21)(
21), 2.644(21)(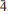 01), 2.296(22)(122). The crystal structures of both minerals were refined by single-crystal X-ray diffraction to R1 = 0.0459 (manganoblödite) and R1 = 0.0339 (cobaltoblödite).
01), 2.296(22)(122). The crystal structures of both minerals were refined by single-crystal X-ray diffraction to R1 = 0.0459 (manganoblödite) and R1 = 0.0339 (cobaltoblödite).
Keywords
- Type
- Research Article
- Information
- Copyright
- Copyright © The Mineralogical Society of Great Britain and Ireland 2013
References
- 15
- Cited by


