Crossref Citations
This article has been cited by the following publications. This list is generated based on data provided by
Crossref.
Braithwaite, R. S. W.
1985.
Roeblingite: a revised formula from infra-red and thermal analysis data.
Mineralogical Magazine,
Vol. 49,
Issue. 354,
p.
756.
Pertlik, F.
and
Zemann, J.
1985.
The crystal structure of scotlandite, PbSO3.
TMPM Tschermaks Mineralogische und Petrographische Mitteilungen,
Vol. 34,
Issue. 3-4,
p.
289.
Paar, W. H.
Mereiter, Kurt
Braithwaite, R. S. W.
Keller, Paul
and
Dunn, P. J.
1986.
Chenite, Pb4Cu(SO4)2(OH)6, a new mineral, from Leadhills, Scotland.
Mineralogical Magazine,
Vol. 50,
Issue. 355,
p.
129.
Nicholson, Keith
and
Banks, David
1987.
Magnetite, pyrrhotine and pentlandite from the Leadhills—Wanlockhead mining district, Scotland.
Mineralogical Magazine,
Vol. 51,
Issue. 359,
p.
175.
Kucha, H.
1988.
Biogenic and non-biogenic concentration of sulphur and metals in the carbonate-hosted ballinalack Zn-Pb deposit, Ireland.
Mineralogy and Petrology,
Vol. 38,
Issue. 3,
p.
171.
Green, David I.
1989.
Scotlandite from Higher Roughton Gill, Caldbeck Fells, Cumbria.
Mineralogical Magazine,
Vol. 53,
Issue. 373,
p.
653.
Kucha, H.
and
Stumpfl, E. F.
1992.
Thiosulphates as precursors of banded sphalerite and pyrite at Bleiberg, Austria.
Mineralogical Magazine,
Vol. 56,
Issue. 383,
p.
165.
Braithwaite, R. S. W.
Kampf, A. R.
Pritchard, R. G.
and
Lamb, R. P. H.
1992.
The occurrence of thiosulfates and other unstable sulfur species as natural weathering products of old smelting slags.
Mineralogy and Petrology,
Vol. 47,
Issue. 2-4,
p.
255.
Weidenthaler, C.
Tillmanns, E.
and
Hentschel, G.
1993.
Orschallite, Ca3(SO3)2 � SO4 � 12H2O, a new calcium-sulfite-sulfate-hydrate mineral.
Mineralogy and Petrology,
Vol. 48,
Issue. 2-4,
p.
167.
Essene, E. J.
Henderson, C. E.
and
Livingstone, A.
2006.
The missing sulphur in mattheddleite, sulphur analysis of sulphates, and paragenetic relations at Leadhills, Scotland.
Mineralogical Magazine,
Vol. 70,
Issue. 3,
p.
265.
Kucha, Henryk
and
Raith, Johann G.
2009.
Gold-oxysulphides in copper deposits of the Greywacke Zone, Austria: A mineral chemical and infrared fluid inclusion study.
Ore Geology Reviews,
Vol. 35,
Issue. 1,
p.
87.
Frost, Ray L.
and
Keeffe, Eloise C.
2009.
Raman spectroscopic study of the sulfite‐bearing minerals scotlandite, hannebachite and orschallite: implications for the desulfation of soils.
Journal of Raman Spectroscopy,
Vol. 40,
Issue. 3,
p.
244.
Pekov, I. V.
Chukanov, N. V.
Britvin, S. N.
Kabalov, Y. K.
Göttlicher, J.
Yapaskurt, V. O.
Zadov, A. E.
Krivovichev, S. V.
Schüller, W.
and
Ternes, B.
2012.
The sulfite anion in ettringite-group minerals: a new mineral species hielscherite, Ca3Si(OH)6(SO4)(SO3)·11H2O, and the thaumasite–hielscherite solid-solution series.
Mineralogical Magazine,
Vol. 76,
Issue. 5,
p.
1133.
Kariper, İ. Afşin
2014.
What is the Effect of Critical Surface Tension of PbSO3 Thin Film?.
Metallurgical and Materials Transactions A,
Vol. 45,
Issue. 10,
p.
4398.
Vignola, P.
Gatta, G. D.
Rotiroti, N.
Gentile, P.
Hatert, F.
Baijot, M.
Bersani, D.
Risplendente, A.
and
Pavese, A.
2016.
Albertiniite, Fe2+(SO3)·3H2O, a new sulfite mineral species from the Monte Falò Pb-Zn mine, Coiromonte, Armeno Municipality, Verbano Cusio Ossola Province, Piedmont, Italy.
Mineralogical Magazine,
Vol. 80,
Issue. 6,
p.
985.
Grundmann, Günter
and
Förster, Hans-Jürgen
2018.
The Sierra de Cacheuta Vein-Type Se Mineralization, Mendoza Province, Argentina.
Minerals,
Vol. 8,
Issue. 4,
p.
127.
Kmak, Kelly N.
Despotopulos, John D.
and
Shaughnessy, Dawn A.
2019.
210Pb/210Po isotope generator.
Journal of Radioanalytical and Nuclear Chemistry,
Vol. 319,
Issue. 1,
p.
257.
Druzhbin, Dmitrii
Rashchenko, Sergey V.
Shatskiy, Anton
and
Crichton, Wilson A.
2022.
New High-Pressure and High-Temperature CaCO3 Polymorph.
ACS Earth and Space Chemistry,
Vol. 6,
Issue. 6,
p.
1506.
Missen, Owen P.
Mills, Stuart J.
Rumsey, Michael S.
Spratt, John
Najorka, Jens
Kampf, Anthony R.
and
Thorne, Brent
2022.
The new mineral tomiolloite, Al12(Te4+O3)5[(SO3)0.5(SO4)0.5](OH)24: A unique microporous tellurite structure.
American Mineralogist,
Vol. 107,
Issue. 12,
p.
2167.
Livingstone, A.
Smith, C.G.
and
MacFadyen, C.C.J.
2022.
Chapter 5 Scottish mineral Geological Conservation Review sites – Hydrothermal veins and mineral assemblages.
Proceedings of the Geologists' Association,
Vol. 133,
Issue. 4-5,
p.
367.
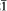 ), 3.07(4) (002), 2.66(7) (020, 012), 2.56(4) (11
), 3.07(4) (002), 2.66(7) (020, 012), 2.56(4) (11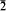 ), 2.24(5) (12
), 2.24(5) (12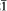 , 102), 2.01(5)(210, 022), 1.707(4) (031), 1.538(4) (032, 004).
, 102), 2.01(5)(210, 022), 1.707(4) (031), 1.538(4) (032, 004).