Crossref Citations
This article has been cited by the following publications. This list is generated based on data provided by
Crossref.
Bottinga, Y
Weill, D
and
Richet, P
1982.
Density calculations for silicate liquids. I. Revised method for aluminosilicate compositions.
Geochimica et Cosmochimica Acta,
Vol. 46,
Issue. 6,
p.
909.
Ghiorso, Mark S.
Carmichael, Ian S. E.
Rivers, Mark L.
and
Sack, Richard O.
1983.
The Gibbs free energy of mixing of natural silicate liquids; an expanded regular solution approximation for the calculation of magmatic intensive variables.
Contributions to Mineralogy and Petrology,
Vol. 84,
Issue. 2-3,
p.
107.
Jeanloz, Raymond
1983.
Mineral and melt physics a summary of research in the United States, 1979–1982.
Reviews of Geophysics,
Vol. 21,
Issue. 6,
p.
1487.
Haggerty, Stephen E.
and
Tompkins, Linda A.
1983.
Redox state of Earth's upper mantle from kimberlitic ilmenites.
Nature,
Vol. 303,
Issue. 5915,
p.
295.
Ghiorso, Mark S
and
Carmichael, Ian S.E
1984.
Comment on “Density calculations for silicate liquids. I. Revised method for aluminosilicate compositions” by Bottinga, Weill and Richet.
Geochimica et Cosmochimica Acta,
Vol. 48,
Issue. 2,
p.
401.
Bottinga, Y.
Weill, D.F.
and
Richet, P.
1984.
Density calculations for silicate liquids: Reply to a Critical Comment by Ghiorso and Carmichael.
Geochimica et Cosmochimica Acta,
Vol. 48,
Issue. 2,
p.
409.
Dick, Henry J. B.
and
Bullen, Thomas
1984.
Chromian spinel as a petrogenetic indicator in abyssal and alpine-type peridotites and spatially associated lavas.
Contributions to Mineralogy and Petrology,
Vol. 86,
Issue. 1,
p.
54.
Arculus, R. J.
Dawson, J. B.
Mitchell, R. H.
Gust, D. A.
and
Holmes, R. D.
1984.
Oxidation states of the upper mantle recorded by megacryst ilmenite in kimberlite and type A and B spinel lherzolites.
Contributions to Mineralogy and Petrology,
Vol. 85,
Issue. 1,
p.
85.
Sparks, R. Stephen J.
and
Huppert, Herbert E.
1984.
Density changes during the fractional crystallization of basaltic magmas: fluid dynamic implications.
Contributions to Mineralogy and Petrology,
Vol. 85,
Issue. 3,
p.
300.
Stebbins, J. F.
Carmichael, I. S. E.
and
Moret, L. K.
1984.
Heat capacities and entropies of silicate liquids and glasses.
Contributions to Mineralogy and Petrology,
Vol. 86,
Issue. 2,
p.
131.
Virgo, David
and
Mysen, Bj�rn O.
1985.
The structural state of iron in oxidized vs. reduced glasses at 1 atm: A57Fe M�ssbauer study.
Physics and Chemistry of Minerals,
Vol. 12,
Issue. 2,
p.
65.
Ghiorso, Mark S.
and
Carmichael, Ian S. E.
1985.
Chemical mass transfer in magmatic processes.
Contributions to Mineralogy and Petrology,
Vol. 90,
Issue. 2-3,
p.
121.
Mysen, Bjorn O.
and
Virgo, David
1985.
Iron-bearing silicate melts: Relations between pressure and redox equilibria.
Physics and Chemistry of Minerals,
Vol. 12,
Issue. 4,
p.
191.
Lowell, Robert P.
1985.
Double-diffusive convection in partially molten silicate systems: Its role during magma production and in magma chambers.
Journal of Volcanology and Geothermal Research,
Vol. 26,
Issue. 1-2,
p.
1.
Mattioli, Glen S.
and
Wood, Bernard J.
1986.
Upper mantle oxygen fugacity recorded by spinel lherzolites.
Nature,
Vol. 322,
Issue. 6080,
p.
626.
Carmichael, Ian S.E.
and
Ghiorso, Mark S.
1986.
Oxidation-reduction relations in basic magma: a case for homogeneous equilibria.
Earth and Planetary Science Letters,
Vol. 78,
Issue. 2-3,
p.
200.
Foley, Stephen F.
Taylor, Wayne R.
and
Green, David H.
1986.
The role of fluorine and oxygen fugacity in the genesis of the ultrapotassic rocks.
Contributions to Mineralogy and Petrology,
Vol. 94,
Issue. 2,
p.
183.
Candela, Philip A
1986.
The evolution of aqueous vapor from silicate melts: Effect on oxygen fugacity.
Geochimica et Cosmochimica Acta,
Vol. 50,
Issue. 6,
p.
1205.
Blake, Stephen
and
Ivey, Gregory N.
1986.
Density and viscosity gradients in zoned magma chambers, and their influence withdrawal dynamics.
Journal of Volcanology and Geothermal Research,
Vol. 30,
Issue. 3-4,
p.
201.
Kelemen, Peter B.
and
Ghiorso, Mark S.
1986.
Assimilation of peridotite in zoned calc-alkaline plutonic complexes: evidence from the Big Jim complex, Washington Cascades.
Contributions to Mineralogy and Petrology,
Vol. 94,
Issue. 1,
p.
12.
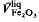 . In a liquid of constant composition and temperature, the pressure dependence of the oxygen fugacity is given by
. In a liquid of constant composition and temperature, the pressure dependence of the oxygen fugacity is given by