No CrossRef data available.
Article contents
Pohlite, a new lead iodate hydroxide chloride from Sierra Gorda, Chile
Published online by Cambridge University Press: 16 November 2022
Abstract
The new mineral pohlite (IMA2022–043), Pb7(IO3)(OH)4Cl9, was found at La Compania mine, Sierra Gorda, Antofagasta Province, Antofagasta, Chile, where it occurs in cavities in an oxidised portion of a quartz vein in association with massive aragonite and anhydrite. Pohlite crystals are transparent, colourless to pale grey blades, up to 4 mm in length. The mineral has a white streak, adamantine lustre and is nonfluorescent. It is brittle with irregular, conchoidal fracture. The Mohs hardness is ~2½ and it has no cleavage. The calculated density is 5.838(2) g cm–3. Optically, the mineral is biaxial (+) with α < 2.01(est.), β = 2.02 (calc.), γ = 2.05 (calc.); 2V = 60(5)°; moderate r > v dispersion; orientation: Y ∧ a ≈ 20°, Z ∧ b ≈ 30°; and is nonpleochroic. The Raman spectrum exhibits bands consistent with IO3– and O–H. Electron microprobe analysis provided the empirical formula Pb6.74I1.00Cl9.29O6.71H4.23. The five strongest powder X-ray diffraction lines are [dobs Å(I)(hkl)]: 3.818(91)(023, 122, 1$\bar{2}$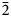 1), 3.674(85)($\bar{1}\bar{2}$
1), 3.674(85)($\bar{1}\bar{2}$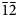 1, $\bar{1}$
1, $\bar{1}$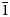 22, 200, 104), 3.399(47)($\bar{2}$
22, 200, 104), 3.399(47)($\bar{2}$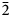 10, 210, $\bar{1}$
10, 210, $\bar{1}$ 04), 2.378(100)(302, 041, $\bar{2}$
04), 2.378(100)(302, 041, $\bar{2}$ 24) and 1.9943(45)(multiple). Pohlite is triclinic, P$\bar{1}$
24) and 1.9943(45)(multiple). Pohlite is triclinic, P$\bar{1}$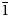 , a = 7.3366(5), b = 9.5130(9), c = 16.2434(15) Å, α = 81.592(7), β = 84.955(7), γ = 89.565(6)°, V = 1117.13(17) Å3 and Z = 2. The structure of pohlite (R1 = 0.0328 for 3394 I > 2σI) contains two types of clusters, a [Pb4(OH)3]5+ cluster formed by short Pb–O bonds and a [Pb3(OH)(IO3)]28+ ‘double cluster’ formed by short I–O bonds and short- to medium-length Pb–O bonds. Long Pb–Cl and I–Cl bonds link the clusters together in three dimensions.
, a = 7.3366(5), b = 9.5130(9), c = 16.2434(15) Å, α = 81.592(7), β = 84.955(7), γ = 89.565(6)°, V = 1117.13(17) Å3 and Z = 2. The structure of pohlite (R1 = 0.0328 for 3394 I > 2σI) contains two types of clusters, a [Pb4(OH)3]5+ cluster formed by short Pb–O bonds and a [Pb3(OH)(IO3)]28+ ‘double cluster’ formed by short I–O bonds and short- to medium-length Pb–O bonds. Long Pb–Cl and I–Cl bonds link the clusters together in three dimensions.
Keywords
- Type
- Article
- Information
- Copyright
- Copyright © The Author(s), 2022. Published by Cambridge University Press on behalf of The Mineralogical Society of Great Britain and Ireland
Footnotes
Associate Editor: David Hibbs


