Published online by Cambridge University Press: 05 July 2018
Qandilite, a new species of the spinel group, has been found in a forsterite-rich rock in contact with a kaersutite-rich banded diorite from the Qala-Dizeh region, NE Iraq. Chemical analysis yielded MgO 29.62, FeO 10.32, MnO 0.76, Fe2O3 28.27, Al2O3 4.83, TiO2 26.41, SiO2 0.02, sum 100.23 wt. %. This analysis calculates to [(Mg1.20Ti0.60)][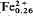 Mg0.12Mn0.02)(
Mg0.12Mn0.02)(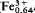 Al0.17)]O4 with a composition of Mg2TiO4 63.8, MgFe2O4 12.8, FeFe2O4 23.4 mole %. Qandilite is cubic with a = 8.4033±0014 Å; cell volume 593.40 Å3 and space group Fd3m; Z = 8. The seven strongest lines in the X-ray diffraction pattern are (d,I,hkl): 2.971, 30, (220); 2.53, 100, (311); 2.101, 45, (400); 1.617, 50, (333, 511); 1.486, 60, (440); 1.0938, 30, (553, 731); 0.9704, 25, (555, 751). The colour is black, lustre metallic, streak black, fracture even and the cleavage perfect on (111). It is opaque, strongly magnetic, Dcalc = 4.04, Dmeas = 4.03, and VHN100 = 960–1045 kg/mm2, with H-7. The reflection colour is grey with a pinkish tint; it is isotopic with no internal reflections. Reflectance measurement data in air and oil in the range (400–700 nm) are given. Colour values for illuminant C in air and oil in terms of CIE colour space are: x = 0.307, 0.307; y = 0.311, 0.310; Y% = 13.10, 3.0; λd = 465, 460; and Pe% = 2.0, 2.2. Calculated data for the absorption coefficients (k) and refractive indices (n) in the range 400–700 nm are also presented.
Al0.17)]O4 with a composition of Mg2TiO4 63.8, MgFe2O4 12.8, FeFe2O4 23.4 mole %. Qandilite is cubic with a = 8.4033±0014 Å; cell volume 593.40 Å3 and space group Fd3m; Z = 8. The seven strongest lines in the X-ray diffraction pattern are (d,I,hkl): 2.971, 30, (220); 2.53, 100, (311); 2.101, 45, (400); 1.617, 50, (333, 511); 1.486, 60, (440); 1.0938, 30, (553, 731); 0.9704, 25, (555, 751). The colour is black, lustre metallic, streak black, fracture even and the cleavage perfect on (111). It is opaque, strongly magnetic, Dcalc = 4.04, Dmeas = 4.03, and VHN100 = 960–1045 kg/mm2, with H-7. The reflection colour is grey with a pinkish tint; it is isotopic with no internal reflections. Reflectance measurement data in air and oil in the range (400–700 nm) are given. Colour values for illuminant C in air and oil in terms of CIE colour space are: x = 0.307, 0.307; y = 0.311, 0.310; Y% = 13.10, 3.0; λd = 465, 460; and Pe% = 2.0, 2.2. Calculated data for the absorption coefficients (k) and refractive indices (n) in the range 400–700 nm are also presented.