Published online by Cambridge University Press: 15 May 2018
Redcanyonite (IMA2016-082), (NH4)2Mn[(UO2)4O4(SO4)2](H2O)4, occurs underground in the Blue Lizard mine, Red Canyon, White Canyon district, San Juan County, Utah, USA. It occurs with natrozippeite, brochantite, devilline, posnjakite, johannite, gypsum, bobcookite, pickeringite, pentahydrite and the NH4-analogue of zippeite: ammoniozippeite. Redcanyonite occurs as radial aggregates of red–orange needles and blades individually reaching up to 0.2 mm long, with aggregates measuring up to 1 mm in diameter. Crystals are flattened on {010} and elongated along [100], exhibit perfect cleavage on {010}, and exhibit the forms {010}, {001}, {101} and {10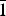 $\bar{1}$}. Twinning is ubiquitous, by 180° rotation on [100]. Redcanyonite is translucent with a pale orange streak, is non-fluorescent, has a Mohs hardness of 2, and has brittle tenacity with uneven fracture. Optically, redcanyonite is biaxial (+), α = 1.725(3), β = 1.755(3), γ = 1.850(5) (white light); 2V (meas.) = 60(2)°, 2V (calc.) = 61.3°; and dispersion is r < v, very strong. Pleochroism is: X = orange, Y = yellow and Z = orange; Y << X < Z. The optical orientation is X = b, Y ≈ c*, Z ≈ a. The empirical formula is (NH4)2.02(Mn0.49Cu0.09Zn0.06)Σ0.64H+0.72[(UO2)4O4(S0.99P0.01O4)2](H2O)4, based on 4 U and 24 O apfu. Redcanyonite is monoclinic, C2/m, a = 8.6572(17), b = 14.155(3), c = 8.8430(19) Å, β = 104.117(18)°, V = 1050.9(4) Å3 and Z = 2. The structure was refined to R1 = 0.0382 for 1079 reflections with Iobs > 3σI. Uranyl oxo-sulfate sheets in redcanyonite adopt the well-known zippeite topology, which consists of zigzag chains of uranyl pentagonal bipyramids linked by sulfate tetrahedra to form sheets. The sheets are linked to each other through bonds to interlayer NH4+ groups and octahedrally coordinated Mn2+, and by hydrogen bonds from H2O groups. Redcanyonite is named for Red Canyon in southeast Utah, USA.
$\bar{1}$}. Twinning is ubiquitous, by 180° rotation on [100]. Redcanyonite is translucent with a pale orange streak, is non-fluorescent, has a Mohs hardness of 2, and has brittle tenacity with uneven fracture. Optically, redcanyonite is biaxial (+), α = 1.725(3), β = 1.755(3), γ = 1.850(5) (white light); 2V (meas.) = 60(2)°, 2V (calc.) = 61.3°; and dispersion is r < v, very strong. Pleochroism is: X = orange, Y = yellow and Z = orange; Y << X < Z. The optical orientation is X = b, Y ≈ c*, Z ≈ a. The empirical formula is (NH4)2.02(Mn0.49Cu0.09Zn0.06)Σ0.64H+0.72[(UO2)4O4(S0.99P0.01O4)2](H2O)4, based on 4 U and 24 O apfu. Redcanyonite is monoclinic, C2/m, a = 8.6572(17), b = 14.155(3), c = 8.8430(19) Å, β = 104.117(18)°, V = 1050.9(4) Å3 and Z = 2. The structure was refined to R1 = 0.0382 for 1079 reflections with Iobs > 3σI. Uranyl oxo-sulfate sheets in redcanyonite adopt the well-known zippeite topology, which consists of zigzag chains of uranyl pentagonal bipyramids linked by sulfate tetrahedra to form sheets. The sheets are linked to each other through bonds to interlayer NH4+ groups and octahedrally coordinated Mn2+, and by hydrogen bonds from H2O groups. Redcanyonite is named for Red Canyon in southeast Utah, USA.
Associate Editor: Giancarlo Della Ventura