Crossref Citations
This article has been cited by the following publications. This list is generated based on data provided by
Crossref.
Sun, Wei
Huang, Ya-Xi
Pan, Yuanming
and
Mi, Jin-Xiao
2016.
Synthesis and magnetic properties of centennialite: a new S = ½ Kagomé antiferromagnet and comparison with herbertsmithite and kapellasite.
Physics and Chemistry of Minerals,
Vol. 43,
Issue. 2,
p.
127.
Mannasova, Alina A.
Chernyatieva, Anastasiya P.
and
Krivovichev, Sergey V.
2016.
Cs2CuP2O7, a novel low-density open-framework structure based upon an augmented diamond net.
Zeitschrift für Kristallographie - Crystalline Materials,
Vol. 231,
Issue. 2,
p.
65.
Sciberras, Matthew J.
Leverett, Peter
Williams, Peter A.
Schlüter, Jochen
Malcherek, Thomas
Welch, Mark D.
Downes, Peter J.
Hibbs, David E.
and
Kampf, Anthony R.
2017.
Structural and compositional variations of basic Cu(II) chlorides in the herbertsmithite and gillardite structure field.
Mineralogical Magazine,
Vol. 81,
Issue. 1,
p.
123.
Krivovichev, Sergey V.
Hawthorne, Frank C.
and
Williams, Peter A.
2017.
Structural complexity and crystallization: the Ostwald sequence of phases in the Cu2(OH)3Cl system (botallackite–atacamite–clinoatacamite).
Structural Chemistry,
Vol. 28,
Issue. 1,
p.
153.
Crichton, Wilson A.
and
Müller, Harald
2017.
Centennialite, CaCu3(OH)6Cl2.nH2O,n≈ 0.7, a new kapellasite-like species, and a reassessment of calumetite.
Mineralogical Magazine,
Vol. 81,
Issue. 5,
p.
1105.
Rossi, Manuela
Rizzi, Rosanna
Vergara, Alessandro
Capitelli, Francesco
Altomare, Angela
Bellatreccia, Fabio
Saviano, Michele
and
Ghiara, Rosaria M.
2017.
Compositional variation of turquoise-group minerals from the historical collection of the Real Museo Mineralogico of the University of Naples.
Mineralogical Magazine,
Vol. 81,
Issue. 6,
p.
1405.
Sushkov, A B
Jenkins, G S
Han, Tian-Heng
Lee, Young S
and
Drew, H D
2017.
Infrared phonons as a probe of spin-liquid states in herbertsmithite ZnCu3(OH)6Cl2.
Journal of Physics: Condensed Matter,
Vol. 29,
Issue. 9,
p.
095802.
Malcherek, Thomas
Mihailova, Boriana
and
Welch, Mark D.
2017.
Structural phase transitions of clinoatacamite and the dynamic Jahn–Teller effect.
Physics and Chemistry of Minerals,
Vol. 44,
Issue. 5,
p.
307.
Nishio–hamane, D.
Momma, K.
Ohnishi, M.
Shimobayashi, N.
Miyawaki, R.
Tomita, N.
Okuma, R.
Kampf, A. R.
and
Minakawa, T.
2017.
Iyoite, MnCuCl(OH)3 and misakiite, Cu3Mn(OH)6Cl2: new members of the atacamite family from Sadamisaki Peninsula, Ehime Prefecture, Japan.
Mineralogical Magazine,
Vol. 81,
Issue. 3,
p.
485.
Mi, Jin-Xiao
and
Pan, Yuanming
2018.
The Role of Halogens in Terrestrial and Extraterrestrial Geochemical Processes.
p.
123.
Inosov, D.S.
2018.
Quantum magnetism in minerals.
Advances in Physics,
Vol. 67,
Issue. 3,
p.
149.
Mercurio, Mariano
Rossi, Manuela
Izzo, Francesco
Cappelletti, Piergiulio
Germinario, Chiara
Grifa, Celestino
Petrelli, Maurizio
Vergara, Alessandro
and
Langella, Alessio
2018.
The characterization of natural gemstones using non-invasive FT-IR spectroscopy: New data on tourmalines.
Talanta,
Vol. 178,
Issue. ,
p.
147.
Mikhailenko, Denis S.
Korsakov, Andrey V.
Rashchenko, Sergey V.
Seryotkin, Yurii V.
Belakovskiy, Dmitriy I.
and
Golovin, Alexander V.
2018.
Kuliginite, a new hydroxychloride mineral from the Udachnaya kimberlite pipe, Yakutia: Implications for low-temperature hydrothermal alteration of the kimberlites.
American Mineralogist,
Vol. 103,
Issue. 9,
p.
1435.
Chukanov, Nikita V.
and
Vigasina, Marina F.
2020.
Vibrational (Infrared and Raman) Spectra of Minerals and Related Compounds.
p.
741.
Crichton, Wilson A.
Müller, Harald
and
Leoni, Matteo
2021.
Synthesis and structure of calumetite-like SrCu4(OH)8Cl2⋅3.5H2O.
Mineralogical Magazine,
Vol. 85,
Issue. 5,
p.
708.
Welch, Mark D.
Najorka, Jens
Rumsey, Michael S.
and
Spratt, John
2021.
The Hexagonal ↔ Orthorhombic Structural Phase Transition in Claringbullite, Cu4FCl(OH)6.
The Canadian Mineralogist,
Vol. 59,
Issue. 1,
p.
265.
Margheri, Simone
Bindi, Luca
Bonazzi, Paola
and
Holtstam, Dan
2022.
Structural and spectroscopic study of well-developed crystals of parahibbingite, β-Fe2(OH)3Cl, formed from terrestrial weathering of the Muonionalusta iron meteorite.
Mineralogical Magazine,
Vol. 86,
Issue. 6,
p.
891.
Kasatkin, Anatoly V.
Siidra, Oleg I.
Nestola, Fabrizio
Pekov, Igor V.
Agakhanov, Atali A.
Koshlyakova, Natalia N.
Chukanov, Nikita V.
Nazarchuk, Evgeny V.
Molinari, Simone
and
Rossi, Manuela
2023.
Napoliite, Pb2OFCl, a new mineral from Vesuvius volcano, and its relationship with dimorphous rumseyite.
Mineralogical Magazine,
Vol. 87,
Issue. 5,
p.
711.
Hallett, Alex
Avarvarei, Catalina
and
Harter, John W.
2023.
Combinatorial exploration of quantum spin liquid candidates in the herbertsmithite material family.
Physical Review Materials,
Vol. 7,
Issue. 6,
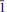 m. Lattice parameters are a = 6.8377(7) Å and c = 14.088(2)Å for the holotype material. The c parameter may vary with Mg/Cu ratio and the presence of impurity atoms. The five strongest lines in the calculated powder diffraction pattern are [d in Å(I)(hkil)]: 5.459(88)(10
m. Lattice parameters are a = 6.8377(7) Å and c = 14.088(2)Å for the holotype material. The c parameter may vary with Mg/Cu ratio and the presence of impurity atoms. The five strongest lines in the calculated powder diffraction pattern are [d in Å(I)(hkil)]: 5.459(88)(10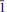 1), 3.419(22)(11
1), 3.419(22)(11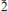 0), 2.764(100)(112 3), 2.266(54)(02
0), 2.764(100)(112 3), 2.266(54)(02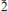 4), 1.706(26)(22
4), 1.706(26)(22 0). Several tondiite crystals have been examined by single-crystal X-ray diffraction and by electron microprobe analysis. The observed Mg content ranges between 0.6 and 0.7 atoms per formula unit. The structural role of Mg is discussed.
0). Several tondiite crystals have been examined by single-crystal X-ray diffraction and by electron microprobe analysis. The observed Mg content ranges between 0.6 and 0.7 atoms per formula unit. The structural role of Mg is discussed.

