Article contents
Vochtenite, (Fe2+,Mg)Fe3+[UO2/PO4]4(OH). 12–13 H2O, a new uranyl phosphate mineral from Wheal Basset, Redruth, Cornwall, England
Published online by Cambridge University Press: 05 July 2018
Abstract
Vochtenite, a new mineral from the Basset Mine, southeast of Camborne in Cornwall, England, is a ferrous-ferric magnesium-bearing hydroxy uranyl phosphate mineral. It is monoclinic with a = 12.606, b = 19.990, c = 9.990 Å, β = 102.31° Z = 3; the ideal formula is:
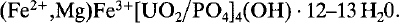
The strongest lines in the X-ray powder diffraction pattern are: 9.998(100)(020), 4.999(30)(040), 4.892(45)(002), 3.475(70)(311), 3.333(50)(060), 3.087(40)(232), 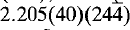 and
and 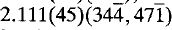 . Vochtenite is brown in colour with a bronzy lustre and is non-fluorescent. Mohs hardness is 2.5 and the density (calc.) = 3.663 g/cm3. There is a prominent (010) cleavage. Vochtenite is optical biaxial negative, 2V (calc.) = 89(3)° its dispersion is indiscernible. Refractive indices are α = 1.575(2), β = 1.589(2), γ = 1.603(2), and the pleochroism is very weak. Orientation X(α)∥b is perpendicular to (010) and Z(γ)∧c is small. The mineral occurs as subparallel (0.5–1.0 mm) crystal aggregates with a pseudo-quadratic outline. The mineral is named vochtenite after Prof. Dr. Ing. R. F. C. Vochten of the State University of Antwerpen, Belgium.
. Vochtenite is brown in colour with a bronzy lustre and is non-fluorescent. Mohs hardness is 2.5 and the density (calc.) = 3.663 g/cm3. There is a prominent (010) cleavage. Vochtenite is optical biaxial negative, 2V (calc.) = 89(3)° its dispersion is indiscernible. Refractive indices are α = 1.575(2), β = 1.589(2), γ = 1.603(2), and the pleochroism is very weak. Orientation X(α)∥b is perpendicular to (010) and Z(γ)∧c is small. The mineral occurs as subparallel (0.5–1.0 mm) crystal aggregates with a pseudo-quadratic outline. The mineral is named vochtenite after Prof. Dr. Ing. R. F. C. Vochten of the State University of Antwerpen, Belgium.
- Type
- Mineralogy
- Information
- Copyright
- Copyright © The Mineralogical Society of Great Britain and Ireland 1989
References
- 2
- Cited by


