Crossref Citations
This article has been cited by the following publications. This list is generated based on data provided by
Crossref.
Siidra, Oleg I.
Vergasova, Lidiya P.
Kretser, Yuri L.
Polekhovsky, Yuri S.
Filatov, Stanislav K.
and
Krivovichev, Sergey V.
2014.
Unique thallium mineralization in the fumaroles of the Tolbachik volcano, Kamchatka Peninsula, Russia. II. Karpovite, Tl2VO(SO4)2(H2O).
Mineralogical Magazine,
Vol. 78,
Issue. 7,
p.
1699.
Siidra, Oleg I.
Vergasova, Lidiya P.
Krivovichev, Sergey V.
Kretser, Yuri L.
Zaitsev, Anatoly N.
and
Filatov, Stanislav K.
2014.
Unique thallium mineralization in the fumaroles of Tolbachik volcano, Kamchatka Peninsula, Russia. I. Markhininite, TlBi(SO4)2.
Mineralogical Magazine,
Vol. 78,
Issue. 7,
p.
1687.
Kovrugin, Vadim M.
Colmont, Marie
Siidra, Oleg I.
Mentré, Olivier
Al-Shuray, Alexander
Gurzhiy, Vladislav V.
and
Krivovichev, Sergey V.
2015.
Oxocentered Cu(ii) lead selenite honeycomb lattices hosting Cu(i)Cl2groups obtained by chemical vapor transport reactions.
Chemical Communications,
Vol. 51,
Issue. 46,
p.
9563.
Aliev, Almaz
Kozin, Michael S.
Colmont, Marie
Siidra, Oleg I.
Krivovichev, Sergey V.
and
Mentré, Olivier
2015.
Synthesis, crystal structure, high-temperature behavior and magnetic properties of CoBiO(AsO4), a Co analogue of paganoite.
Physics and Chemistry of Minerals,
Vol. 42,
Issue. 8,
p.
663.
Kovrugin, Vadim M.
Siidra, Oleg I.
Colmont, Marie
Mentré, Olivier
and
Krivovichev, Sergey V.
2015.
Emulating exhalative chemistry: synthesis and structural characterization of ilinskite, Na[Cu5O2](SeO3)2Cl3, and its K-analogue.
Mineralogy and Petrology,
Vol. 109,
Issue. 4,
p.
421.
Pekov, Igor V.
Siidra, Oleg I.
Chukanov, Nikita V.
Yapaskurt, Vasiliy O.
Britvin, Sergey N.
Krivovichev, Sergey V.
Schüller, Willi
and
Ternes, Bernd
2015.
Engelhauptite, KCu3(V2O7)(OH)2Cl, a new mineral species from Eifel, Germany.
Mineralogy and Petrology,
Vol. 109,
Issue. 6,
p.
705.
Krivovichev, Sergey V.
2017.
Structure description, interpretation and classification in mineralogical crystallography.
Crystallography Reviews,
Vol. 23,
Issue. 1,
p.
2.
Pekov, Igor V.
Siidra, Oleg I.
Yapaskurt, Vasiliy O.
Polekhovsky, Yury S.
and
Kartashov, Pavel M.
2018.
Ziminaite, Fe3+VO4, a new howardevansite-group mineral from the Bezymyannyi volcano, Kamchatka, Russia.
Mineralogy and Petrology,
Vol. 112,
Issue. 3,
p.
371.
Siidra, Oleg I.
Nazarchuk, Evgeny V.
Agakhanov, Atali A.
and
Polekhovsky, Yury S.
2019.
Aleutite [Cu5O2](AsO4)(VO4)·(Cu0.5□0.5)Cl, a new complex salt-inclusion mineral with Cu2+substructure derived from a Kagome-net.
Mineralogical Magazine,
Vol. 83,
Issue. 6,
p.
847.
Siidra, Oleg I.
Nazarchuk, Evgeny V.
Zaitsev, Anatoly N.
Polekhovsky, Yury S.
Wenzel, Thomas
and
Spratt, John
2019.
Dokuchaevite, Cu8O2(VO4)3Cl3, a new mineral with remarkably diverse Cu2+ mixed-ligand coordination environments.
Mineralogical Magazine,
Vol. 83,
Issue. 5,
p.
749.
Pekov, I. V.
Zubkova, N. V.
Yapaskurt, V. O.
Koshlyakova, N. N.
Turchkova, A. G.
Sidorov, E. G.
and
Pushcharovsky, D. Yu.
2020.
Polymorphism and Isomorphic Substitutions in the $${\text{Cu}}_{3}^{{2 + }}$$(T
5+O4)2 Natural System with T = As, V, or P.
Geology of Ore Deposits,
Vol. 62,
Issue. 8,
p.
803.
Siidra, Oleg I.
Vladimirova, Victoria A.
Tsirlin, Alexander A.
Chukanov, Nikita V.
and
Ugolkov, Valery L.
2020.
Cu9O2(VO4)4Cl2, the First Copper Oxychloride Vanadate: Mineralogically Inspired Synthesis and Magnetic Behavior.
Inorganic Chemistry,
Vol. 59,
Issue. 4,
p.
2136.
Pekov, Igor V.
Zubkova, Natalia V.
Yapaskurt, Vasiliy O.
Polekhovsky, Yury S.
Britvin, Sergey N.
Turchkova, Anna G.
Sidorov, Evgeny G.
and
Pushcharovsky, Dmitry Yu.
2020.
Kainotropite, Cu4Fe3+O2(V2O7)(VO4), a new mineral with a complex vanadate anion from fumarolic exhalations of the Tolbachik volcano, Kamchatka, Russia.
The Canadian Mineralogist,
Vol. 58,
Issue. 2,
p.
155.
Zhitova, E. S.
Anikin, L. P.
Sergeeva, A. V.
Ismagilova, R. M.
Rashidov, V. A.
Chubarov, V. M.
and
Kupchinenko, A. N.
2021.
Volborthite Occurrence at the Alaid Volcano (Atlasov Island, Kuril Islands, Russia).
Geology of Ore Deposits,
Vol. 63,
Issue. 7,
p.
735.
Kornyakov, Ilya V.
Vladimirova, Victoria A.
Siidra, Oleg I.
and
Krivovichev, Sergey V.
2021.
Expanding the Averievite Family, (MX)Cu5O2(T5+O4)2 (T5+ = P, V; M = K, Rb, Cs, Cu; X = Cl, Br): Synthesis and Single-Crystal X-ray Diffraction Study.
Molecules,
Vol. 26,
Issue. 7,
p.
1833.
Shablinskii, Andrey P.
Avdontceva, Margarita S.
Vergasova, Lidiya P.
Filatov, Stanislav K.
Avdontseva, Evgenia Yu.
Povolotskiy, Alexey V.
Moskaleva, Svetlana V.
Kargopoltsev, Anatoly A.
Britvin, Sergey N.
and
Shorets, Olga U.
2022.
Medvedevite, KMn2+V5+2O6Cl⋅2H2O, a new fumarolic mineral from the Tolbachik fissure eruption 2012–2013, Kamchatka Peninsula, Russia.
Mineralogical Magazine,
Vol. 86,
Issue. 3,
p.
478.
Kiriukhina, G. V.
Yakubovich, O. V.
Dimitrova, O. V.
and
Volkov, A. S.
2022.
New Microporous Copper Diphosphate Chloride in a Series of Homeotypic Compounds: Hydrothermal Synthesis, Crystal Structure, and Crystal Chemistry.
Crystallography Reports,
Vol. 67,
Issue. 4,
p.
545.
Ginga, Victoria A.
Siidra, Oleg I.
Firsova, Vera A.
Charkin, Dmitri O.
and
Ugolkov, Valery L.
2022.
Phase evolution and temperature-dependent behavior of averievite, Cu5O2(VO4)2(CuCl) and yaroshevskite, Cu9O2(VO4)4Cl2.
Physics and Chemistry of Minerals,
Vol. 49,
Issue. 9,
Kornyakov, Ilya V.
and
Krivovichev, Sergey V.
2022.
Na2Cu+[Cu2+
3O](AsO4)2Cl and Cu3[Cu3O]2(PO4)4Cl2: two new structure types based upon chains of oxocentered tetrahedra.
Zeitschrift für Kristallographie - Crystalline Materials,
Vol. 237,
Issue. 8-9,
p.
343.
Chukanov, Nikita V.
Fischer, Reinhard X.
Kazheva, Olga N.
and
Aksenov, Sergey M.
2023.
Gladstone–Dale compatibility, electronic polarizability and vibrational spectroscopy of minerals and inorganic compounds with V4+O and V4+O2 vanadyl groups.
Acta Crystallographica Section B Structural Science, Crystal Engineering and Materials,
Vol. 79,
Issue. 4,
p.
336.
 , a = 6.4344(11), b = 8.3232(13), c = 9.1726(16) Å , α = 105.338(14), β = 96.113(14), γ = 107.642(1)°, V = 442.05(13) Å3 and Z = 1. The nine strongest reflections in the X-ray powder pattern [dobs in Å (I)(hkl)] are as follows: 8.65(100)(001); 6.84(83)(0
, a = 6.4344(11), b = 8.3232(13), c = 9.1726(16) Å , α = 105.338(14), β = 96.113(14), γ = 107.642(1)°, V = 442.05(13) Å3 and Z = 1. The nine strongest reflections in the X-ray powder pattern [dobs in Å (I)(hkl)] are as follows: 8.65(100)(001); 6.84(83)(0 1); 6.01(75)(100); 5.52(60)(
1); 6.01(75)(100); 5.52(60)( 01); 4.965(55)(011); 4.198(67)(
01); 4.965(55)(011); 4.198(67)(
 1); 4.055(65)(110); 3.120(55)(021); 2.896(60)(2
1); 4.055(65)(110); 3.120(55)(021); 2.896(60)(2 1,003,
1,003,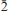 20). The crystal structure was solved by direct methods from single-crystal X-ray diffraction data and refined to R = 0.0737. The yaroshevskite structure is unique. It is based on corrugated layers made up of chains of edge-sharing flat squares with central Cu2+ cations [Cu(1), Cu(4) and Cu(5)]; neighbouring chains are connected via groups consisting of three Cu2+ -centred squares [two Cu(3) and Cu(6)]. Neighbouring layers are connected via pairs of Cu(2)O4Cl five-coordinate polyhedra and isolated VO4 tetrahedra. The structure of yaroshevskite can also be considered in terms of oxygen-centred tetrahedra: O(7)Cu4 tetrahedra are connected via common Cu(4) and Cu(5) vertices to form pyroxene-like chains [O2Cu6]∞. In this context, the structural formula can be written Cu3[O2Cu6][VO4]4Cl2. The mineral name honours the Russian geochemist Alexei A. Yaroshevsky (b. 1934) of Moscow State University.
20). The crystal structure was solved by direct methods from single-crystal X-ray diffraction data and refined to R = 0.0737. The yaroshevskite structure is unique. It is based on corrugated layers made up of chains of edge-sharing flat squares with central Cu2+ cations [Cu(1), Cu(4) and Cu(5)]; neighbouring chains are connected via groups consisting of three Cu2+ -centred squares [two Cu(3) and Cu(6)]. Neighbouring layers are connected via pairs of Cu(2)O4Cl five-coordinate polyhedra and isolated VO4 tetrahedra. The structure of yaroshevskite can also be considered in terms of oxygen-centred tetrahedra: O(7)Cu4 tetrahedra are connected via common Cu(4) and Cu(5) vertices to form pyroxene-like chains [O2Cu6]∞. In this context, the structural formula can be written Cu3[O2Cu6][VO4]4Cl2. The mineral name honours the Russian geochemist Alexei A. Yaroshevsky (b. 1934) of Moscow State University.

