Introduction
The availability of a material for technological purposes is, in practice, defined not only by its abundance in Earth’s crust, but also by the amount of energy necessary for its extraction from ores. The latter factor, although frequently overlooked, is often decisive. Therefore, in this article, we discuss the thesis that the energy costs of critical materials can severely limit the transition from existing technologies to new ones. This thesis includes three parts that we view as critical in considering materials–energy interdependence:
(1) There is very little flexibility in the ability to divert energy resources to new technologies.
(2) Production of materials that are currently obtained as byproducts of other, more prominent materials cannot be increased rapidly, a fact that imposes severe restrictions on the rate of technology change.
(3) Recycling can provide only partial relief of the demand for energy to produce materials, because many items that are energy-intensive to make and/or use (not just sophisticated items such as spectacles and contact lenses, but also rather basic high-volume items such as fertilizer and cement) cannot be recycled. Moreover, recycling of some items might consume excessive amounts of energy.
Difficulties estimating the true energy cost of materials
The energy cost of a material is complicated, as it balances the amounts of energy used (or recovered) during the material’s production, use, and ultimate disposal or recycling. A steel-frame building provides a good illustration of this tradeoff. Use of a steel frame is one of the most energy-efficient methods of construction; however, steel is a very good thermal conductor, which makes such a building more expensive to heat and cool.Footnote *, 1–Reference Winistorfer, Chen, Lippke and Stevens5 Calculating the energy cost of production of materials is not easy, but a methodology based on the general principle of conservation of energy and matter is available, because it has been “inherited” from chemical engineering calculations and is actively used for life-cycle assessment and net energy analysis (see, for example, References Reference Oloman6 and Reference Hirschberg, Roth, Bauer, Burgherr, Dones, Heck and Schenler7). In contrast, calculating or measuring the energy consumed during use is not trivial because there are many contributions that are difficult or even impossible to estimate correctly. Therefore, the total energy cost of a certain material cannot be determined very accurately, at least at present. However, even if reliable estimates were available, their use in material–energy efficiency planning would be possible only after one considered the flexibility of allocation of energy resources.
Five “major” energy-consuming materials and the flexibility of energy consumption
More than half (∼55%)8 of global energy consumption is by industry and transportation (∼27.5% each).8, 9 The other half is by services, residential consumers, and infrastructure. The energy used by industry is mostly for materials processing. Remarkably, comparison of the energy costs of materials production shows that more than half of all industrial energy use goes to a very small number of materials, namely, steel (∼6%),10, Reference Scrivener and Van Damme11 cement (∼3%; see the article by Van Vliet et al. in this issue),Reference West12 ammonia13, 14 by the Haber–Bosch process (1–2%), aluminum by the Hall–Héroult process (∼1.8%),10, Reference Scrivener and Van Damme11 and plastics (2–4%).Reference Scrivener and Van Damme11, Reference Kuhlke and Walsh15 It is also important to emphasize that, although new “high-tech” materials have penetrated almost all parts of modern technologies (e.g., titanium, magnesium, tungsten carbide, mu-metal, industrial diamond, conducting and antistatic plastics), they account for a very small fraction of the materials used in everyday life.
These five major energy-consuming materials are, together with the other top energy consumer, transportation, vital for daily life, and therefore, their production (and consumption) cannot be restricted without an immediate and drastic drop in living standards. This implies that, at the current stage, society has restricted its ability to divert energy resources anywhere else on a large scale, leaving only residential use (24% of the total) and commercial services (<10%) as major sources for such a diversion, should it become necessary. One could also expect some contribution from transportation. However, unless there is a mass transition from private cars to public transport or a dramatic drop in cargo shipments, transportation will not be able to contribute significantly. Therefore, based on the variations in materials and energy consumptions that have occurred during periods of economic crisis, when people tend to cut the least essential expenditures, one can “guesstimate” that at most ∼10% of these energy uses (i.e., about 3.5% of total global energy production) can be diverted to a transition to alternative technologies without disrupting living standards in a major way.
How can sustainable energy availability affect sustainable materials availability?
The very close connection between materials and energy suggests that changes in the availability of one energy source will immediately cause changes in the availability of all other energy sources and, in that way, cause a ripple throughout the system, affecting all materials. At the same time, the analysis in the preceding section indicates that diversion of a few percent of total energy resources for “transition purposes” is very likely possible. Because the range of materials in practical use is restricted by the amount of energy required for their production, a rapid rise in energy costs will inevitably be accompanied by a reduction in (or even the complete elimination of) the use of materials with high energy costs. If changes are sufficiently gradual, as many economists (and others) predict, then the adaptation of materials production to changes in energy sources will also be smooth, and the materials in use will adapt to the new restrictions on energy availability. Examples of such smooth transitions include the replacements of blubber by kerosene for lighting in the late 19th century and of Bakelite by modern polyethylene-based plastics after the development of the latter in the 1950s.
If changes were rapid, which could happen if, for example, oil supply dropped by 50% within a few months, then many materials would rapidly disappear from use because of the jump in energy prices, causing severe disruptions. The degree of these disruptions would depend on how fast an alternative energy source could be deployed. In some cases, such alternatives exist now, but their deployment is obstructed for various reasons. An example of such a case is the possible replacement of gasoline by methanol (see the sidebar).16, 17 Although such a replacement seems to be viable already with the current wholesale prices, it has not yet taken place for political reasons that are beyond the scope of this article and issue. Similar economically feasible or almost feasible alternatives exist for many (if not all) energy-production technologies. Thus, even with rapid changes and severe disruptions, one can expect that adaptation will eventually take place.
However, the example with methanol involves two hidden assumptions: that the changes in energy availability will be on a scale of a few percent of the actual energy consumption and that the materials necessary for a transition to a new energy-generation technology will be available. Although the first condition is almost guaranteed because oil wells will not go dry instantaneously and coal fields will not be exhausted at once, the second condition is questionable, as discussed in detail in the next section.
Availability of materials produced as byproducts
Apart from a relatively large but finite list of materials that are produced and extracted directly from ores (so-called primary products, such as iron, copper, aluminum, and tin), many materials are extracted as byproducts of a primary product. For instance, selenium and tellurium are byproducts of the electrolytic refining of copper, during which they accumulate in anode residues. The total annual production is ∼2300–2500 t of selenium (2009) and ∼150 t of tellurium (2010).10 Significantly increasing the production of these two elements using the existing technological route is not an option in the short term (a few years), because it would require a many-fold increase of copper production, which is not viable, either practically or economically. Production of refined copper, excluding its transport, has an energy price of ∼23 GJ/t,18 which is increasing steadily as high-grade ores become exhausted and hauling distances increase. One can expect rising prices to be an incentive for more efficient extraction, but even in the best scenarios, increased efficiency is unlikely to increase the production several-fold.
The case of photovoltaics illustrates the dilemma of depending on materials that are byproducts. Whereas the article by Fthenakis in this issue discusses the availability of materials for new solar cells, we focus here on the energy required to acquire the materials. For the sake of argument, assume that we want to achieve a 100-fold increase in materials supply, from today’s 0.07 TWp (terawatt-peak) installed generating capacity to 7 TWp, which corresponds to ∼1.15 TWc (terawatt-continuous, assuming optimal use of the generating capacity).
For the increasingly popular CdTe solar cell, this increased energy production would require a 100-fold increase in tellurium production, assuming current efficiencies. In the following discussion, for the sake of simplicity, we assume that all of the new tellurium goes to CdTe solar cell production. In that case, a 100-fold increase in copper production would be required. In 2009, copper production (∼16 Mt)10, 18 used about 0.08% of all global energy. Increasing this value 100-fold is hardly realistic, because it would consume all of the “flexible” part of the available energy defined in the preceding section. Furthermore, it would require construction for primary copper ore treatment on a huge scale, which is not a realistic proposition, especially within a period of a few years.
The situation is very similar for gallium and indium, required for Cu(In,Ga)Se2 (CIGS) solar cells. Both of these elements are byproducts of the production of other elements. Gallium is mostly produced from residues of bauxite (aluminum ore) or extracted from zinc-processing residues. In both cases, the gallium content does not exceed 50 ppm, and its annual total production is ∼180 t (2008). Furthermore, even though the total content of gallium in known deposits of bauxites and zinc ores is ∼1 Mt, a 100-fold increase of gallium production would require that all energy currently used for industrial consumption be directed to gallium production. To install 0.01 TWc capacity based on CIGS solar cells would require more than 105 t of gallium, so that a 100-fold increase might not suffice (again, assuming that all new gallium went to CIGS cell production). The value of this example is that it demonstrates a material (gallium) that is known to exist and to have an accessible extraction technology, but for which the extraction energy requirements are prohibitively high. In answer to the question posed at the beginning of this article, then, this implies that sustainable energy availability and sustainable materials availability are not equivalent. In practice, the term “energy availability” always refers to some reference value, which is typically the current level of consumption. One of the most obvious consequences of this conclusion is that, as things stand now, the world in ∼2040 will not be able to rely on these compound solar cells for, say, 5% of global electricity generation (∼1–2 TWc, which requires a global average of 6–12 TWp) with present types of cells and present mining and extraction technologies.
Now consider the energetic viability of crystalline-silicon solar cells. In contrast to the thin-film cells just discussed, in these cells, the raw material is plentiful and is obtained as a primary product. For crystalline-silicon solar cells, the energy payback time, which is the time required for the cells to produce the amount of energy needed to make them, is still several years.19
Because of this long payback time, one can expect that, even if 1% of all energy for industrial use (0.25% of the total use) were diverted to create silicon solar cells, issues such as borrowing costs and return-on-investment times would impede the rapid manufacturing of all of the cells needed for this extent of electrical power generation. Also, even if ways are found to decrease the energy payback time (e.g., metallurgical refinement of silicon in place of silane-based purification), it will take decades for silicon cells to make a significant contribution to total global energy production. Nevertheless, this shows that, after a few decades, a transition to solar power based on crystalline silicon is energetically feasible. (Other problems associated with the technology, such as land availability and dust control, will also need to be solved.)
As a final example, consider the production of hydrogen through water electrolysis using platinum-based electrodes. The total amount of platinum produced each year from ores (not recycled) is ∼180 t.10 Assume that one-tenth of this total production is diverted to water electrolysis. Then, running a cell at a most optimistic 1.5 V potential (82% efficiency), using 100-nm-thick electrodes and restricting the current to 0.1 A/cm2 to minimize platinum gas erosion, one could convert 135 GW of electrical energy into hydrogen. This is less than 0.3% of the energy required for transportation. Even if such a diversion of platinum continued for 50 years, which is unlikely because platinum is needed for other purposes, hydrogen would still not be an important transportation fuel. The scale of platinum extraction that would be needed to support a major portion of global fuel needs would require prohibitively high energy diversion. Nickel-based catalysts can be used instead of platinum, but at present, their use exacts a significant increase in energy price. Moreover, a sizable increase in platinum production is not feasible at present, because the platinum content of the richest known ore (Buchveld, South Africa, responsible for more than 75% of world production) is only ∼8–9 g/t (4–7 g/t is more common for platinum ores).Footnote ‡ Surely, one can use electrodes other than platinum; however, this would result in a considerable efficiency loss (more than a factor of two) and require correspondingly higher amounts of energy.
Even for common materials, energy availability must be considered, as a sudden increase in usage could cause significant upheaval. For instance, if cement production had to be increased by one-third, a 1% increase in total world energy production would be required. Although possible, this increase would be hard to achieve within a short time and would severely strain society’s ability to undertake other large-scale projects.
Two conclusions can be drawn from the analyses in this section: (1) The idea that sustainable availability of energy is equivalent to sustainable availability of materials is true only in the long run. In the short run of a few years, any increase in the production of byproducts (secondary mining products) is essentially impossible. (2) Some materials are physically unavailable at any energy price in the quantity needed or desired.
Energy efficiency and the potential of recycling
One often hears that, with time, production becomes less materials- and energy-intensive, because of the introduction of increasingly efficient processes. Although this trend is generally valid, production of materials might prove to be an exception for a number of reasons, the most obvious of which is the depletion of rich ores (although there are different opinions on this matterReference Mudd21, Reference West22). Increased hauling distances are also a factor, as is becoming increasingly evident, for instance, for copper production.18 In this view, the impact of energy costs on materials availability results from more than one trend. Therefore, the question of whether consumption of materials will continue to grow or stabilize becomes clearly linked to the monetary cost of energy (and to the energy cost of the forms of energy needed). As noted by Krausmann et al., global materials extraction increased rapidly (close to exponentially) during the past 100 years (although much of this increase comes from increasing population; the increase in materials use per capita was much more modest),Reference Krausmann, Gingrich, Eisenmenger, Erb, Haberl and Fischer-Kowalski23 as shown in Figure 1. Therefore, both the total amount of energy used by industry and the industrial fraction of total energy consumption have increased steadily and will continue to do so.

Figure 1. (a) Total annual materials use per year and (b) annual materials use per capita. Data and figure used with permission from Reference Reference Krausmann, Gingrich, Eisenmenger, Erb, Haberl and Fischer-Kowalski23. © 2009, Elsevier.
One of the main, and probably most efficient, ways to alleviate this trend is recycling. (See the article in this issue by Gaines.) Currently, metals are recycled at reasonable rates, with the following fractions of recycled metals in new products: lead, >90%; iron, 55–65%; aluminum, 40–50%; tin, >50%; magnesium, >40%; and copper, >25%.24 Naturally, these are materials with high to very high energy price tags, and one can expect their degrees of recycling to continue to increase, although it will never reach 100%. However, two large contributors to industrial energy use cannot be efficiently recycled, even in theory, namely, cement and fertilizer. Taken together, these materials comprise about 4–5% of global energy consumption and about 20–25% of global industrial energy consumption (see the section Five “major” energy-consuming materials and the flexibility of energy consumption). With continuing increases in standard of living, production and consumption (Figure 1)Reference Krausmann, Gingrich, Eisenmenger, Erb, Haberl and Fischer-Kowalski23 of these materials will continue to rise, and according to current trends, within the next few decades, one can expect these materials to become even more dominant energy consumers than they are now. Furthermore, as natural (as opposed to cultivated) food resources dwindle (for instance, caught rather than farmed fish) and the number of cultivated products increases, the amount of energy required for engineered replacements will continue to grow.Reference Erb, Krausmann, Gaube, Gingrich, Bondeau, Fischer-Kowalski and Haberl25 Thus, although extremely important, recycling will not be a universal cure for energy savings, and the price of energy will continue to influence everyday life significantly.
In conclusion, materials availability is indeed limited by energy availability. However, because the amount of energy that can be diverted for transitioning to new technologies at any given moment is limited, sustainable availability is not equivalent for these two entities. This thesis is of the utmost importance for making decisions about which types of alternative technologies are to be adopted, because current prices and current energy expenditures might not reflect those that will be relevant if even a small increase in demand occurs. Indeed, it is possible that, even after a transition is initiated, materials unavailability could render its completion impossible.
Methanol versus gasoline prices
Price of methanol on 3 August 2011: US$459/t
Density of methanol: 0.79 g/cm3
Ratio of methanol to gasoline energy content (w/w): 0.55
Cost of the amount of methanol equivalent to 1 gal (U.S.) of gasoline:
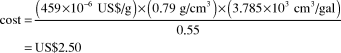
CostFootnote † of 1 gal (U.S.) of gasoline before taxes on 3 August 2011: US$3.20




