Cyanoacrylate, which is better known as Super Glue, is the most commonly used product to bind two clean surfaces of different chemistries. Adhesion occurs by the polymerization of acrylate monomers in the presence of water molecules from the environment. However, this gluing effect is severely hindered in highly hydrated conditions. Other strategies therefore need to be developed for sticking wet hydrogels together or to hydrophobic surfaces such as stretchy elastomers. The team of Zhigang Suo at Harvard University, with collaborators at Zhejiang University, has addressed this issue and brings a new strategy for manufacturing soft elastomer-hydrogel-based devices for soft robotics or biomimetic applications.
The new adhesion method, which was published in Nature Communications (doi:10.1038/s41467-018-03269-x), works as follows: silane coupling agents (Si–O–X bond) are added directly to the precursor mixtures of both the hydrogel and the elastomer to be glued. In the presence of water and during the formation of the polymer networks, the silanes hydrolyze into silanol (Si–O–H), which then condense into siloxane bonds (Si–O–Si). One key discovery is that “to bond elastomers and hydrogels, the interfacial bonding density only needs to be comparable to the cross-linking density. Since the cross-linking density can be as low as 1 per 1000 monomers, strong bonding actually requires very little interfacial bonding. Our interface is strong because we match the interfacial bonding density with the bulk cross-linking density,” says Qihan Liu, lead author of the paper.
Better than traditional glues, the interface between the two materials occurs at the mesh size level and can reach adhesion energies up to 866 J/m2, as measured by a peeling test. This value, which was obtained for a silicone (PDMS) attached to a toughened hydrogel of polyacrylamide (PAAm), is nearly three orders of magnitude higher than between noncovalently bond elastomer hydrogels. In addition to giving strong adhesion, the strategy is versatile. The amounts of the silanes and cross-linker can be independently varied in order to tune the mechanics of each polymer, their adhesion, and the kinetics of the respective reactions. The addition of small fractions of surfactant or an increase in temperature can also provide an efficient means of reducing the total amount of silanes by concentrating them at the interface.
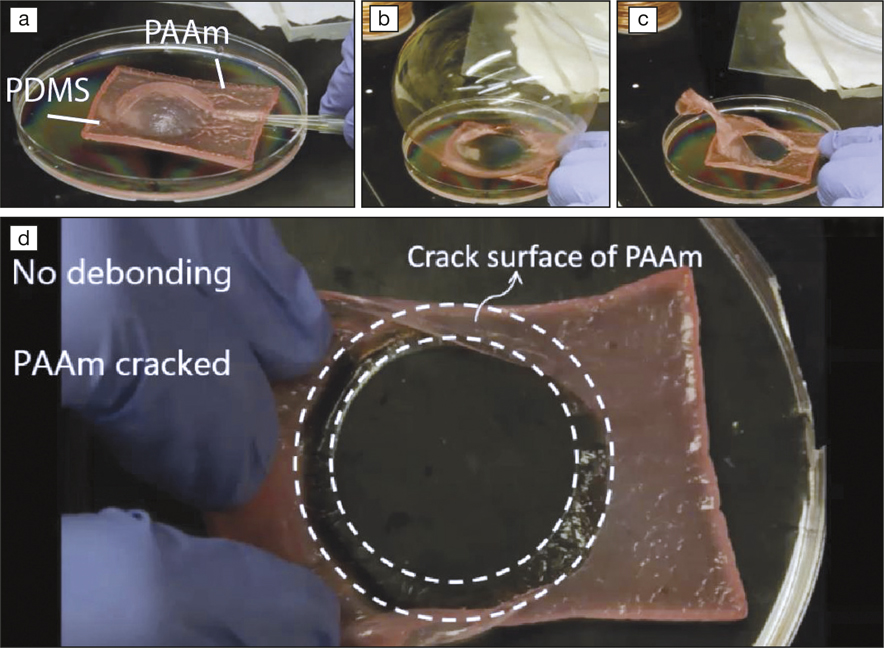
(a–c) Polyacrylamide (PAAm) silane-modified hydrogel bound to a polydimethylsiloxane (PDMS) silane-modified elastomer along a ring. By inserting a nozzle between the two layers, the PAAm membrane is inflated into a balloon. (d) After the balloon burst, no debonding is observed at the interface. Instead, cracks occur in the hydrogel. Credit: Adapted from Nature Communications.
This multimaterial binding strategy is unique in that it can be readily applied to a large variety of manufacturing processes. Alba Marcellan, associate professor at Sorbonne Université (ex-UPMC) in Paris and leading her research on gel adhesion and mechanics at ESPCI Paris, explains that “based on their remarkable features of absorption, storage or release of water, gels have become essential in engineering applications like super-absorbents, soil-less agriculture, or tissue engineering. Gels are also envisaged to be key players for the design of flexible actuators, valves, or sensors. However, their generally weak mechanical performance combined with the inherent difficulties of manufacturing and assembling them still limit industrial development.” Suo’s team has developed a method similar to “autogenous welding.” “I am really impressed by the method in terms of manufacturing capabilities: gluing, casting, printing, coating.... In this last example, an extremely thin and compliant elastomer coating can be applied on the gel and allows for working under isolated conditions, avoiding exchanges between the hydrogel and the environment, but also to make the gel surface wear-resistant,” Marcellan says.
With the potential of many applications, Liu and his colleagues are now fabricating multilayered electrode-insulator structures by alternatively dip-coating hydrogel and elastomer, to create new soft and stretchable electroluminescent objects of arbitrary shapes.


