The core of a perovskite solar cell is a perovskite active layer sandwiched between an electron- and a hole-transporting layer. The hole-transport materials that yield high-efficiency solar cells could limit commercialization: they are expensive and require dopants that are hygroscopic, causing devices to degrade. “It is difficult to achieve both high efficiency and good stability,” says Jangwon Seo of the Korea Research Institute of Chemical Technology.
Seo, Jun Hong Noh, and their colleagues have resolved this issue. They have made efficient and stable solar cells using dopant-free poly(3-hexylthiophene) (P3HT) as the hole-transport material. P3HT has excellent optoelectronic properties and is low cost and easy to make using simple printing techniques. But perovskite solar cells made with it have only reached efficiencies of 16%.
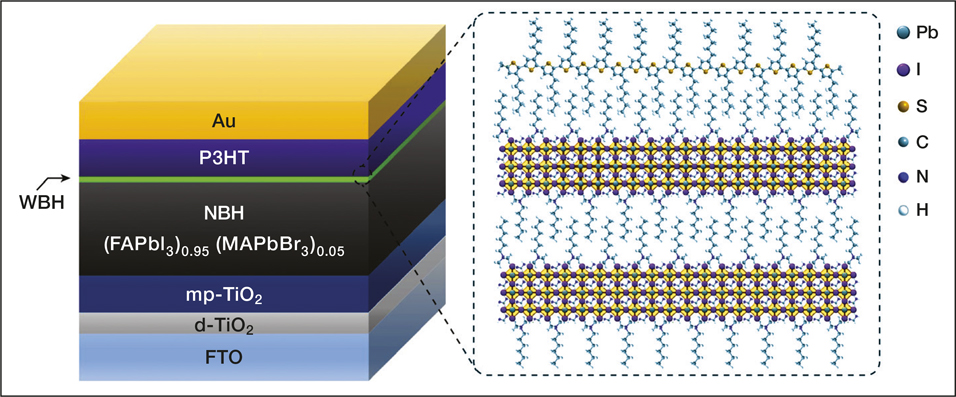
(Left) The structure of an n-i-p perovskite solar cell based on a double-layered halide architecture using P3HT as the hole-transport material. WBH, wide-bandgap halide; NBH, narrow-bandgap halide; mp-TiO2, mesoporous titanium dioxide; d-TiO2, dense titanium dioxide; FTO, fluorine-doped tin oxide. (Right) Schematic structure of the interface between the WBH and P3HT. Credit: Nature.
The researchers designed a double-layered device architecture in which they stacked an ultrathin wide-bandgap layer onto a narrow-bandgap light-absorbing material. They made the wide-bandgap layer simply by the reaction of n-hexyl trimethyl ammonium bromide (HTAB) on a formamidinium lead iodide and meth-ylammonium lead bromide perovskite surface. “The hexyl chain in HTAB, present at the surface of the double-layered hal-ide perovskite, could interact favorably with the hexyl chain in P3HT via van der Waals [bonds] for better interfacial contact, promoting self-assembled P3HT nanofibrils that result in considerably improved hole extraction,” explains Seo.
The device, reported in the journal Nature (doi:10.1038/s41586-019-1036-3) had an efficiency of 22.7% and when encapsulated maintained 95% of this initial efficiency for 1370 h under 1-sun illumination at room temperature.
One way to boost the power-conversion efficiency and open-circuit voltage of perovskite solar cells is to suppress defects in the perovskite material that can trap charge carriers, making it easier for them to recombine. Researchers at the Chinese Academy of Sciences have come up with an efficient, simple method to tackle defects: an organic halide salt that can be applied to mixed perovskite polycrystalline films to quench defects. They were able to make perovskite solar cells with a record-high certificated efficiency of 23.32%.
Defects most easily form on the surface, and researchers have recently tried various halide compounds to passivate the defects in the perovskite layer. Jingbi You and colleagues found that the organic halide salt phenethylammonium iodide (PEAI) can form on a perovskite surface and works better than the layered PEA2PbI4 perovskite proposed in many recent studies to reduce the defects. This work appeared in Nature Photonics (doi:10.1038/s41566-019-0398-2).
Solar cells made with perovskites that have mixed cations boast some of the best power-conversion efficiencies. Adding alkali metals such as cesium and rubidium to the organic cations methylammonium or formamidinium improves device performance and stability, but researchers have not understood why.
A study published in Science (doi:10.1126/science.aah5065) illuminates how adding alkali metal cations to halide perovskites improves their electronic properties. The results indicate that there is an optimal concentration level for these additives.
Researchers from Georgia Institute of Technology, Massachusetts Institute of Technology, and the University of California, San Diego, used synchrotron-based nanoscale x-ray fluorescence to map the elemental composition of the perovskites and analyze them at the nanoscale. They found that adding cesium and rubidium to a lead-halide perovskite caused the halides to mix more homogeneously, improving charge-carrier lifetime and mobility and resulting in up to 2% higher conversion efficiency than materials without these additives. But that only worked to a certain level. When the alkali metal concentration increased over 1%, the metals formed clusters where charge carriers could recombine and decrease the material’s conductivity.
A study in Energy & Environmental Science (doi: 10.1039/c8ee03051k) offers key insights into the stability of mixed-cation perovskites under operating conditions. The results show that stability not only depends on device architecture and chemical formulation, the perovskite processing temperature and strategy are critically important in making stable device systems. A team led by Laura Schelhas at the SLAC National Accelerator Laboratory and Joseph Berry at the National Renewable Energy Laboratory used in situ x-ray diffraction to observe how structural stability evolved across APbI3 perovskites, where A is a combination of formamidinium, Cs, or methylammonium. “The binary CsFA material was susceptible to phase segregation, but the ternary material was much more stable during operation,” Schelhas says. The researchers used thermodynamic calculations to observe the relationship between the material composition and processing temperature for “how strongly the material wants to be mixed.”
This shows that processing temperature is important for creating a well-mixed material, Schelhas adds. “If the material is not well mixed initially, it will be more susceptible to phase segregation or de-mixing, which can kill the overall device performance.”


