With the rapid expansion of technology comes an increasing demand for new and improved functional materials. Developing materials for a wide variety of applications, such as solar cells, transistors, light-emitting devices (LEDs), fuel cells, energy storage, and drug delivery is crucial for advancing our everyday life. One overlooked tool for measurements of materials is time-resolved optical spectroscopy, which is mainly used by physical chemists today. Time-resolved optical spectroscopy offers materials researchers and engineers powerful methods for analyzing materials in action. A stimulus, which is often (but not limited to) a pulse of light, initiates a dynamical process in a material that is tracked over time by meas-uring its interaction either with or through the emission of light (Figure 1).
Figure 1. Simplified illustration of a time-resolved optical spectroscopy experiment. A stimulus induces a change in the sample that is monitored by measuring its interaction with light.
But what do spectroscopists actually do, and how can they contribute to materials science? A spectroscopist studies how molecules and materials interact with light. Measuring their specific interactions yields information about the material. Often, a spectroscopist is interested in solving fundamental problems; however, time-resolved optical spectroscopy can also be applied to measure materials functionality over time. For example, it can be used to watch electrons migrate in a solar cell or energy flow within an LED before it produces light. It can be used to watch chemical bonds break or form, or monitor structural organization of polymers in thin films. Such information is particularly useful for designing and testing a new material and can help solve contemporary problems in materials science and engineering.
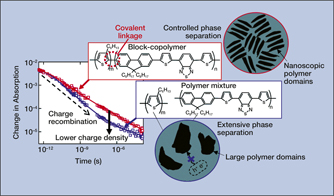
For instance, transient absorption spectroscopy (TAS) is a time-resolved method that is used to monitor excited species that form and decay in a material following light absorption. A particularly appealing application of TAS is to study charge generation and recombination in solar-energy-conversion materials, such as organic semiconducting polymers and inorganic perovskites. TAS measurements of charge-carrier recombination were used in combination with x-ray scattering to explain how the chemical structure in conjugated block-copolymer materials can be tailored to optimize thin-film nanomorphology and enhance their performance in solar cells.Reference Grieco, Aplan, Rimshaw, Lee, Le, Zhang, Wang, Milner, Gomez and Asbury1Figure 2 illustrates how more charges survive in the block-copolymer material compared to a polymer mixture, because the latter is able to undergo nanoscale self-assembly. This process limits phase separation, enabling light-generated, bound charges to transfer and separate at the interfaces between the polymer domains.
Other exciting opportunities include multidimensional spectroscopy techniques, such as two-dimensional infrared (2D IR) spectroscopy, which have become significantly more accessible because of continually improving technological advances. The 2D IR technique probes molecular-level vibrational motion to obtain structural and dynamical information about a material, which is useful for making connections between materials design and performance. In the past, instrumentation required rigorous maintenance, and measurements were very time-consuming. Now 2D IR meas-urements are applied more routinely, leading to an acceleration in our understanding of materials with functions ranging from biology to energy conversion and storage.Reference Petti, Lomont, Maj and Zanni2
Previous challenges to implementing time-resolved optical spectroscopy included limited awareness and practicality. In particular, the language of spectroscopists is different from that of materials scientists and engineers, so the benefits of spectroscopic tools are not readily discerned by the latter. In addition, time-resolved spectroscopy was traditionally difficult to carry out. But now with improved laser, optical, and detector technologies, it is becoming a much more accessible, standardized, and affordable tool. New technologies are emerging and continuing to improve, such as fully integrated transient absorption instruments like a UV–vis or Fourier transform infrared spectroscopy, which are becoming easier to use.
What remains is to merge spectroscopy with engineering and materials research and to expand time-resolved optical spectroscopy into a versatile tool for analyzing and guiding materials development. This would also foster multidisciplinary approaches for solving problems. But how can we facilitate this? We can start by providing additional multidisciplinary sessions at professional scientific conferences for exposing problems faced in a variety of scientific fields; contemporary research is commonly exposed in sessions segregated by discipline. Physical chemists can also publish more perspective or tutorial articles on applying spectroscopy to materials systems.
Figure 2. Transient absorption kinetic decays of charge carriers that form in polymer films upon light absorption. Charge recombination is tracked over time, revealing a lower charge density for the polymer mixture at later times. The cartoons illustrate phase separation of the polymer domains that occurs in the films.
Developing new functional materials is becoming increasingly important. The fusion of materials synthesis, fabrication, and characterization with time-resolved optical spectroscopy would be a huge step toward advancing the technologies of our everyday world.
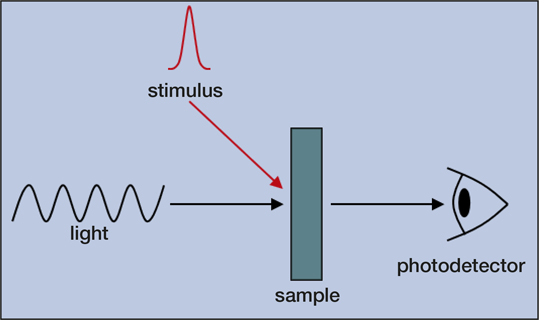



With the rapid expansion of technology comes an increasing demand for new and improved functional materials. Developing materials for a wide variety of applications, such as solar cells, transistors, light-emitting devices (LEDs), fuel cells, energy storage, and drug delivery is crucial for advancing our everyday life. One overlooked tool for measurements of materials is time-resolved optical spectroscopy, which is mainly used by physical chemists today. Time-resolved optical spectroscopy offers materials researchers and engineers powerful methods for analyzing materials in action. A stimulus, which is often (but not limited to) a pulse of light, initiates a dynamical process in a material that is tracked over time by meas-uring its interaction either with or through the emission of light (Figure 1).
Figure 1. Simplified illustration of a time-resolved optical spectroscopy experiment. A stimulus induces a change in the sample that is monitored by measuring its interaction with light.
But what do spectroscopists actually do, and how can they contribute to materials science? A spectroscopist studies how molecules and materials interact with light. Measuring their specific interactions yields information about the material. Often, a spectroscopist is interested in solving fundamental problems; however, time-resolved optical spectroscopy can also be applied to measure materials functionality over time. For example, it can be used to watch electrons migrate in a solar cell or energy flow within an LED before it produces light. It can be used to watch chemical bonds break or form, or monitor structural organization of polymers in thin films. Such information is particularly useful for designing and testing a new material and can help solve contemporary problems in materials science and engineering.
For instance, transient absorption spectroscopy (TAS) is a time-resolved method that is used to monitor excited species that form and decay in a material following light absorption. A particularly appealing application of TAS is to study charge generation and recombination in solar-energy-conversion materials, such as organic semiconducting polymers and inorganic perovskites. TAS measurements of charge-carrier recombination were used in combination with x-ray scattering to explain how the chemical structure in conjugated block-copolymer materials can be tailored to optimize thin-film nanomorphology and enhance their performance in solar cells.Reference Grieco, Aplan, Rimshaw, Lee, Le, Zhang, Wang, Milner, Gomez and Asbury1Figure 2 illustrates how more charges survive in the block-copolymer material compared to a polymer mixture, because the latter is able to undergo nanoscale self-assembly. This process limits phase separation, enabling light-generated, bound charges to transfer and separate at the interfaces between the polymer domains.
Other exciting opportunities include multidimensional spectroscopy techniques, such as two-dimensional infrared (2D IR) spectroscopy, which have become significantly more accessible because of continually improving technological advances. The 2D IR technique probes molecular-level vibrational motion to obtain structural and dynamical information about a material, which is useful for making connections between materials design and performance. In the past, instrumentation required rigorous maintenance, and measurements were very time-consuming. Now 2D IR meas-urements are applied more routinely, leading to an acceleration in our understanding of materials with functions ranging from biology to energy conversion and storage.Reference Petti, Lomont, Maj and Zanni2
Previous challenges to implementing time-resolved optical spectroscopy included limited awareness and practicality. In particular, the language of spectroscopists is different from that of materials scientists and engineers, so the benefits of spectroscopic tools are not readily discerned by the latter. In addition, time-resolved spectroscopy was traditionally difficult to carry out. But now with improved laser, optical, and detector technologies, it is becoming a much more accessible, standardized, and affordable tool. New technologies are emerging and continuing to improve, such as fully integrated transient absorption instruments like a UV–vis or Fourier transform infrared spectroscopy, which are becoming easier to use.
What remains is to merge spectroscopy with engineering and materials research and to expand time-resolved optical spectroscopy into a versatile tool for analyzing and guiding materials development. This would also foster multidisciplinary approaches for solving problems. But how can we facilitate this? We can start by providing additional multidisciplinary sessions at professional scientific conferences for exposing problems faced in a variety of scientific fields; contemporary research is commonly exposed in sessions segregated by discipline. Physical chemists can also publish more perspective or tutorial articles on applying spectroscopy to materials systems.
Figure 2. Transient absorption kinetic decays of charge carriers that form in polymer films upon light absorption. Charge recombination is tracked over time, revealing a lower charge density for the polymer mixture at later times. The cartoons illustrate phase separation of the polymer domains that occurs in the films.
Developing new functional materials is becoming increasingly important. The fusion of materials synthesis, fabrication, and characterization with time-resolved optical spectroscopy would be a huge step toward advancing the technologies of our everyday world.