Published online by Cambridge University Press: 09 October 2017
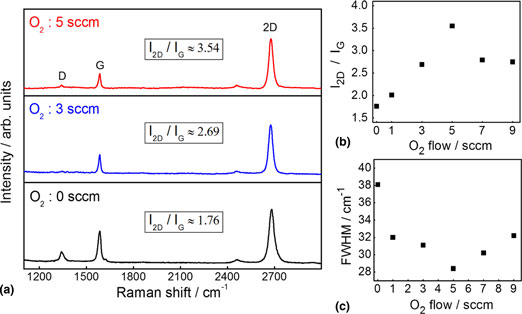
Chemical vapor deposition is the most proficient method for growing graphene on copper foils due to its scalability, repeatability, and uniformity, etc. Herein, we systematically study the effect of oxygen (O2) exposure on graphene growth. We introduced O2 before and during the growth, and then studied its effects on the morphology, crystallinity, and nucleation density of graphene. We observe that introducing O2 during growth significantly improves the graphene crystallinity while pre-dosing O2 before growth reduces the graphene nucleation density. These studies suggest that intermittent O2 exposure play a significant role in graphene growth, enabling scalable production of high-quality graphene.