Article contents
Emerging trends in bioenergy harvesters for chronic powered implants
Published online by Cambridge University Press: 22 June 2015
Abstract
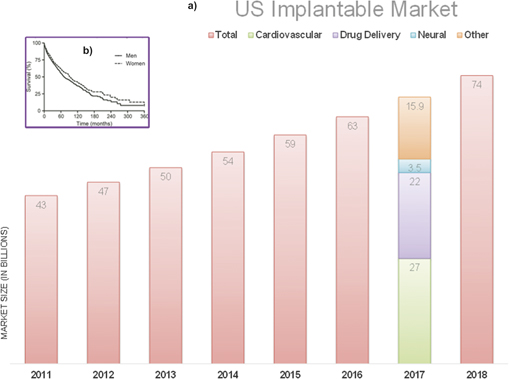
The widening gap between the short battery life (<8 years) and patients' life expectancy (20 years) is a growing concern for long-term implantable devices and adds to outpatient costs. This gap coupled with significant advancements in circuit, device design, and lowered power consumption (<1 mW) has refueled the interest in implantable energy harvesters.
As the complexity of implantable devices is increasing, the size and power requirements of implantable devices have shrunk by more than double over the past few decades. However, the functionality or lifespan of the devices is often found to be limited due to shortage of power. With more than 50% of the device size being occupied by the battery alone, longevity of such implantable devices has garnered huge concern over the years. Fueled by the demand of additional biosensors coupled to such devices, implantable energy harvesters, capable of harvesting the body's chemical, thermal, or mechanical energy over a long period of time, have gained tremendous popularity. Among these technologies, implantable glucose fuel cells provide a promising method to generate a small yet continuous supply of power. Implantable fuel cells tap into the available free blood glucose to generate electricity. With the trend moving toward the use of semiconductor technologies for glucose-based fuel cells, fabrication of reliable and effective technology is within feasible limits. Realization of such implantable power sources can shift the burden from commonly used lithium-ion batteries by utilizing physiological resources. The present review focuses on recent developments on abiotic glucose fuel cell for bioenergy harvesting.
- Type
- Review
- Information
- Copyright
- Copyright © Materials Research Society 2015
References
REFERENCES
- 9
- Cited by


