Article contents
Determination of Diffusion Parameters for Arsenic
Published online by Cambridge University Press: 25 February 2011
Abstract
This paper deals with an analysis of the oxynitridation and direct nitridation experiments of Fahey et al.(1984[1]). The inconsistencies in the evaluation of the fiactional component of diffusion for aisenic are discussed. Using a recently developed diffusion model an explanation for the diffusion data for arsenic is given. The standard equation 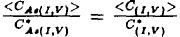 for analyzing diffusion under nonequilibrium conditions is shown to lead to erroneous results. A consistent treatment must account for the concentrations of arsenic, point defects and arsenic point defect pairs. Pair formation kinetics must be included. Values are presented for the diffusion constants DIAs, DVAS, and the reaction constants kIAs, kVAs depending on the fractional interstitial component of diffusion
for analyzing diffusion under nonequilibrium conditions is shown to lead to erroneous results. A consistent treatment must account for the concentrations of arsenic, point defects and arsenic point defect pairs. Pair formation kinetics must be included. Values are presented for the diffusion constants DIAs, DVAS, and the reaction constants kIAs, kVAs depending on the fractional interstitial component of diffusion 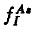 .
.
- Type
- Research Article
- Information
- Copyright
- Copyright © Materials Research Society 1990
References
- 2
- Cited by


