1. Introduction
Adolescents (10–19 years old) represent 1·2 billion individuals worldwide mostly clustered in low-income and middle-income countries(Reference Lansford and Banati1). Adolescent global health and nutrition have been poorly evaluated (Reference Christian and Smith2,Reference Mokdad, Forouzanfar and Daoud3) and health services have struggled to address health needs(4). Adolescence was added in 2016 into the Global Strategy report as an essential component to achieve the Sustainable Development Goals(5). This showed the need to account for adolescents ‘because they are central to everything we want to achieve, and to the overall success of the 2030 Agenda, (pg. 5)’. Adolescence is a vulnerable period but also a window of opportunity to address physical and mental health, catch up with growth and ensure improved future outcomes(Reference Banati and Camilletti6). It is also a period of nutritional burden facing undernutrition as well as overnutrition (overweight and obesity)(4). Lifestyle, eating behaviours, underlying psychosocial factors and socio-economic determinants are key to adolescent health and nutrition(4).
Factors associated with adolescent undernutrition are complex and involve determinants at individual, household and population level(Reference Patton, Sawyer and Santelli7). The World Health Organization (WHO) identifies some underlying causes of adolescent undernutrition such as diet, diseases, injuries, infections and early pregnancy(8). Undernutrition is assessed by measuring height and weight as well as examining for clinical manifestations and biochemical markers(9). Stunting, thinness and micronutrient deficiencies are forms of adolescent undernutrition(10). Stunting reflects chronic undernutrition, and it is associated with poor cognitive development, lower school performance and reduced economic productivity(Reference Cashin and Oot11). Stunting is defined as height-for-age 2 SDs below the WHO Child Growth Reference median(Reference de Onis, Onyango and Borghi12). Thinness or underweight in adolescents is associated with a higher risk of infectious diseases, delayed maturation, reduced muscular strength, work capacity and bone density later in life. In addition, thinness in adolescent girls is associated with adverse pregnancy outcomes and intra-uterine growth retardation(13). Thinness is defined as BMI-for-age 2 SDs below the WHO Growth Reference median(Reference de Onis, Onyango and Borghi12). Micronutrient deficiencies indicate a lack of essential vitamins and minerals required in small amounts by the body for adequate growth and development(Reference Ritchie and Roser14).
Three hundred and forty million adolescents live in South Asia, accounting for 30% of the world adolescent population(15). South Asian adolescents are still widely invisible with minimal access to evidence based information and restricted decision-making on issues affecting their life, especially among girls(16). Adolescent girls are more likely to be anaemic, experience gender discrimination, have a poor education and health, and suffer exploitation and violence. The UNICEF reports that 11% of South Asian adolescent girls aged 15–19 years are stunted, 39% are thin and 55% are anaemic(16).
Previous reviews are mainly focused on younger children(Reference Khan, Bano and Salam17–Reference Pasricha and Biggs19) or do not include all forms of adolescent undernutrition(Reference Mak and Tan20) in South Asia. A comparison of these studies was published elsewhere(Reference Estecha Querol, Al-Khudairy and Iqbal21), revealing the need for a more systematic approach to synthesise the evidence. This scoping review will include a large pool of evidence by following a broad search strategy including all forms of undernutrition as well as including grey literature without restrictions on date of publication. Following the Population, Concept and Context (PCC) strategy(Reference Peters, Godfrey and McInerney22), we aimed to answer the following question: what do we know about adolescent undernutrition in South Asia from the existing literature? Therefore, this scoping review aims to map the evidence on adolescent undernutrition in South Asia and identify gaps in knowledge.
2. Methods
The protocol of this review was previously published(Reference Estecha Querol, Al-Khudairy and Iqbal21). The methodology of this scoping review was informed by Arksey and O’Malley’s framework(Reference Arksey and O’Malley23) and The Joanna Briggs Institute Reviewers’ Manual(Reference Peters, Godfrey and McInerney22). In addition, we followed the Preferred Reporting Items for Systematic reviews and Meta-Analyses extension for Scoping Reviews (PRISMA-ScR) checklist where applicable(Reference Tricco, Lillie and Zarin24). Since a scoping review involves a methodical integration and presentation of available resources, this study does not require ethics approval.
2.1. Inclusion and exclusion criteria
Studies were included if participants were adolescents with a mean age 10–19 years old(25) from Afghanistan, Bangladesh, Bhutan, India, Maldives, Nepal, Pakistan and Sri Lanka(26).
Studies also needed to investigate undernutrition as its primary outcome. Adolescent undernutrition indicators include (1) thinness or underweight (low BMI-for-age), (2) stunting (low height-for-age) and (3) micronutrient deficiencies (lack of essential vitamins and minerals required in small amounts by the body for proper growth and development(Reference Ritchie and Roser14)). Studies were included regardless of the growth references or cut-off values followed. Qualitative studies exploring perspectives, experiences or opinions around adolescent undernutrition were included. All study designs of peer-reviewed journals as well as grey literature were included. No restrictions on language or publication date were made. Studies were excluded if they focused on overnutrition indicators alone (obesity and overweight), pregnant or breast-feeding adolescents, adolescent athletes, adolescents with long-term conditions such as diabetes, tuberculosis or HIV, hospitalised adolescents or intervention studies targeting treatment of a specific illness or condition such as diarrhoea.
2.2. Methods for identifying relevant studies
The search was conducted using Medline (OVID), Embase, Cochrane, Web of Science, CINAHL, PsycInfo, Scopus, the WHO Library Information System (WHOLIS), eLENA e-Library of Evidence for Nutrition Actions, and Opengrey. The websites of relevant agencies, academic institutions and technical bodies were also searched: WHO, UNICEF, Demographic and Health Surveys (DHS), Program, Planning and Development Department AJ&K, Global Health Data Exchange (GHDx), World Food Program (WFP) and World Bank eLibrary. Searches were carried out from inception to 11 March 2019. The search strategy included South Asia AND adolescents AND undernutrition. Terms related to undernutrition were also considered in the search strategy. The final search strategy for all databases can be found in the published protocol(Reference Estecha Querol, Al-Khudairy and Iqbal21).
2.3. Selection process and data extraction
Firstly, titles and abstract were reviewed by two independent reviewers (S.E.Q. and M.K.) following a broad inclusion criterion, that is, studies looking at adolescent undernutrition in South Asia. Papers identified by either or both reviewers were included in the next phase. Secondly, two reviewers independently (S.E.Q. and N.O.) screened full-text studies using the eligibility criteria mentioned above, and reasons for exclusion were documented. At this stage, disagreements were resolved by either discussion or referral to a third reviewer (L.A.K.).
A form was developed and piloted to extract relevant information of the included studies (available in the published protocol(Reference Estecha Querol, Al-Khudairy and Iqbal21)). One reviewer (S.E.Q.) extracted data. A second reviewer (L.A.K.) reviewed the results of data charting to resolve any conflict and ensure consistency. For all included studies, key characteristics were charted using an Excel sheet. We extracted data on study characteristics (e.g. author(s)/organisation, year of publication, location, design, and sample size), target population characteristics (e.g. age, sex and setting), adolescent undernutrition indicators (stunting, thinness, and micronutrient deficiencies) and undernutrition outcomes (e.g. prevalence).
We reported the results as a data map presented in a tabular form displaying distribution of publication year, location, characteristics of target population, study design and outcomes. Additionally, a narrative synthesis accompanied the descriptive presentation.
3. Results
3.1. Results of the search
The search located 6972 records from databases and 1103 records from grey literature such as websites of agencies, academic institutions and technical bodies (Fig. 1). De-duplication resulted in a total of 6181 records that were screened. Title and abstract screening excluded 5640 records. Out of the 541 full-text records assessed for eligibility, 401 did not meet the inclusion criteria. A total of 140 records (131 publications) met the inclusion criteria of this review. Records were grouped under publications if they belonged to the same research project, reporting the group of records as one publication. There were three systematic reviews that were screened for relevant publications. No further evidence was identified from these systematic reviews. Therefore, a total of 128 publications were formally included in this review constituting of 108 original articles, ten letters to editor, six reports from international agencies, three conference abstracts and one communication.

Fig. 1. Selection of sources of evidence in this review using the original PRISMA statement(Reference Moher, Liberati and Tetzlaff97).
3.2. Characteristics of the included studies
Information on the author, year of publication, study title, age and sex of the target population, location, malnutrition indicator(s), classification, growth standards and main findings is provided for the 128 eligible publications (Supplementary material 1). The Supplementary material 1 table categorises the literature into two main groups: one where an intervention component is present (n = 12) and one where it is not (n = 116).
The majority of the eligible evidence was published in the last 15 years (Table 1). The first publication measuring micronutrient deficiencies was in 1975(Reference Tandon, Ramachandran and Nath27), the first publication measuring stunting was in 1996(Reference PanterBrick, Todd and Baker28) and the first publication measuring thinness was in 2002(Reference Venkaiah, Damayanti, Nayak and Vijayaraghavan29). All included publications reported quantitative outcomes (Table 1). Eligible evidence involved nine randomised controlled trials, two longitudinal studies, one non-randomised prospective trial, one pre–post interventional study and 115 cross-sectional studies (Table 1).
Table 1. Characteristics of the included studies

3.2.1. Geographical distribution of the evidence
The majority of publications (67%) were conducted in India (Table 1). The geographical distribution of the literature was aggregated in some of the states or provinces of Bangladesh, India, Nepal, Pakistan and Sri Lanka (Fig. 2). Evidence from Bhutan, Afhanistan and Maldives was obtained solely from the Global School-based Student Health Survey(30–32). The setting was only reported in 76 publications (60%) (Table 1), of which 32 were conducted in rural areas (25%), 8 in slum areas (7%), 14 in urban areas (11%), 10 in both urban and rural areas (8%), and 12 were nationally representative samples (9%). Sixty-nine of the included publications were school-based (53%), and 34 were community-based (35%) (Table 1).
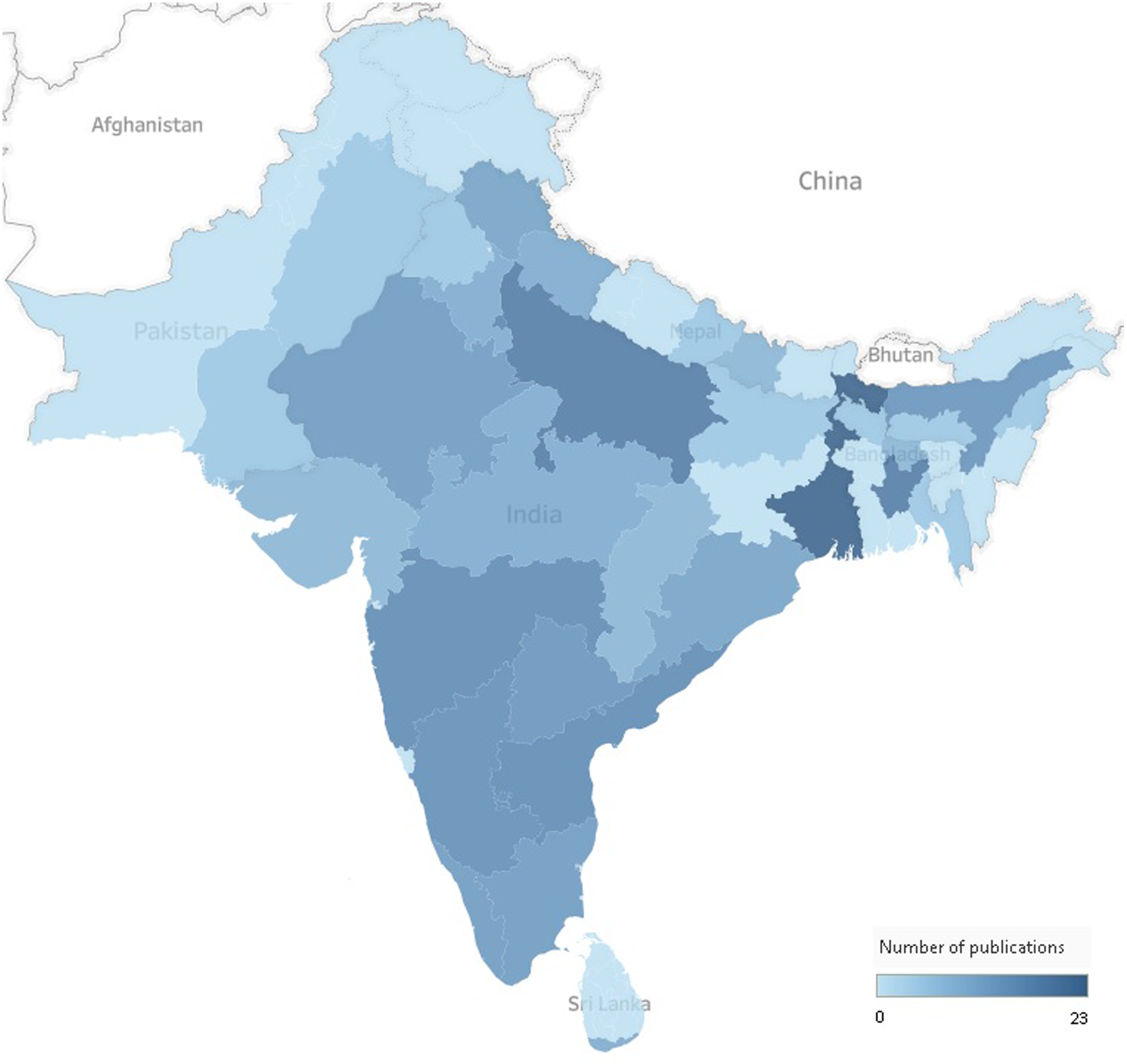
Fig. 2. Geographical distribution of the literature across South Asian countries. The total number does not equal the total number of publications included in the scoping review as some publications focused on more than one country, state or province.
3.2.2. Target population
Sex-stratified analysis was provided in 77 publications (60%) (Table 1). Forty-five publications reported data on girls only, and six publications included boys only. In terms of participants’ age, 82 publications focused exclusively on the age range of 10–19 years. Sixteen publications had a mean age of 10–19 years. In publications where the mean age was greater than 10–19 years, data regarding adolescents were only extractable in 30 publications. The sample size was extracted from 122 publications (95%). The sample size ranged from 41 to 16 245, with a mean of 1704 adolescents. In six publications, it was not possible to extract the sample size because it was not reported. Adolescents were anaemic at baseline in four publications(Reference Prakash, Prakash, Sharma and Pal33–Reference Ahmed, Khan, Banu, Qazi and Akhtaruzzaman36).
3.2.3. Undernutrition indicators
Thinness (low BMI-for-age) was frequently (n = 70) used to measure adolescent undernutrition. Stunting (low height-for-age) was used as an undernutrition indicator in 54 publications, and micronutrient deficiency was also reported in 55 publications (Table 1).
Twenty-seven publications categorised adolescent nutritional status using age- and sex-specific anthropometric measures using National Center for Health Statistics (NCHS) growth charts (Supplementary material 1). Other growth references considered appropriate to assess BMI-for-age or height-for-age were WHO reference 2007 (n = 22), Centers for Disease Control and Prevention (CDC) growth charts (n = 11), National Health and Nutrition Examination Survery (NHANES) (n = 11), WHO reference 1995 (n = 9), and Indian Academy of Pediatrics (IAP) growth charts (n = 5). Ten publications used International Obesity Task Force (IOTF) criteria on sex-age-specific BMI cut-offs for thinness, and three publications used Waterlow’s classification of stunting. The citations of the listed growth references are provided in Supplementary material 1.
Anaemia was the most reported sign of a micronutrient deficiency (Table 2). It was reported in 38 publications, of which 20 referenced the WHO classification of low haemoglobin concentration. Other micronutrient deficiencies examined were vitamin A (n = 13), folic acid (n = 9), vitamin B12 (n = 7), vitamin D (n = 6), vitamin C (n = 5), zinc (n = 5), vitamin B2 (n = 3), iodine (n = 3) and iron (n = 2).
Table 2. Micronutrient deficiencies and indicators reported

3.2.4. Publications that included an intervention component
Results of publications that include an intervention component (n = 12) are presented in Supplementary material 1. A brief on findings of these publications is summarised below.
Among the publications that include an intervention component, supplementation was the most prevalent type of intervention, using food fortification in six publications and capsules in five. In addition, there was one school feeding program which provided daily balanced meals to a cohort of tribal students attending a school in rural south India(Reference Thomas, Srinivasan and Sudarshan37).The authors of this publication classified the students according to attendance: ‘new’ students less than 1 year and ‘old’ students more than 1 year of attendance. Comparison of thinness prevalence in these groups showed that 50% of ‘new’ and 37·5% of ‘old’ students were thin; however, the difference was not significantly different between the two groups.
In Sri Lanka, iron and zinc capsules consumed daily on school days for 24 weeks showed improvements in the prevalence of anaemia and zinc deficiency but did not decrease the rate of stunting among adolescents(Reference Hettiarachchi, Liyanage, Wickremasinghe, Hilmers and Abrams38). Various publications compared the efficacy of different micronutrient supplementation combinations among anaemic adolescents(Reference Prakash, Prakash, Sharma and Pal33–Reference Ahmed, Rahman Khan and Akhtaruzzaman35) and vitamin D deficient adolescents(Reference Garg, Marwaha and Khadgawat39). A sample of 178 anaemic adolescent schoolgirls in Bangladesh received iron–folic acid – 30 mg Fe and 400 µg folic acid – tablets or multiple micronutrients (MMN) tablets (15 micronutrients, including iron and folic acid)(Reference Ahmed, Rahman Khan and Akhtaruzzaman35). The findings showed that even though the effect of iron–folic acid tablets and MMN tablets on reducing anaemia and iron deficiency was similar, MMN supplementation demonstrated a significant enhancement of other micronutrients’ status. The team examined the relative efficacy of long-term (52 weeks) once-weekly and twice-weekly MMN supplementation(Reference Ahmed, Khan and Akhtaruzzaman34). Both supplementation frequencies were equally efficacious in decreasing anaemia; however, twice-weekly MMN was more efficacious than once-weekly MMN in improving vitamin A, riboflavin and folic acid status. Another randomised controlled trial studied the effect of non-iron containing ayurvedic preparations to treat nutritional anaemia in adolescent students in India(Reference Prakash, Prakash, Sharma and Pal33). The authors found that a daily dose of two non-iron containing ayurvedic preparations improved anaemia in the study participants. An intervention providing 1500 μg/week doses of vitamin D to vitamin D deficient adolescents revealed that 4-, 6- and 8-week supplementation regimens were equally efficacious in achieving vitamin D sufficiency(Reference Garg, Marwaha and Khadgawat39). Micronutrient-fortified biscuits confirmed to be effective in improving not only micronutrient deficiencies but also thinness and stunting among Indian adolescent girls(Reference Goyle40). Evidence showed that food fortification is a cost-effective and sustainable strategy to improve micronutrient deficiencies among adolescents in Bangladesh(Reference Rahman, Ahmed, Ahmed, Alam, Wahed and Sack41) and India(Reference Vir, Singh, Nigam and Jain42), respectively. School-based interventions showed that vitamin D fortification(Reference Khadgawat, Marwaha and Garg43) and iron fortification(Reference Muthayya, Thankachan and Hirve44) was an effective strategy for wider use in school feeding programs. School-based supplementation programs also resulted in higher adherence due to supervision(Reference Hyder, Haseen and Khan45). However, a project providing weekly iron–folic acid tablets to adolescent girls did not find any difference in the impact on anaemia prevalence between school girls (supervised) and non-school girls (unsupervised)(Reference Vir, Singh, Nigam and Jain42).
3.2.5. Publications that did not include an intervention component
We identified 116 publications that assessed nutritional status, that is, the prevalence of undernutrition. Among these, six publications compared stunting and/or thinness prevalence using different growth references, and 38 publications assessed undernutrition risk factors such as age, sex, demographic determinants and socio-economic status. Results of publications that did not include an intervention component are displayed in Supplementary material 1, and a narrative summary is provided below.
The prevalence of stunting, thinness and micronutrient deficiencies was challenging to compare because the included publications followed different growth references. Prevalence varied widely and is presented in Supplementary material 1. Prevalence of stunting ranged from 7%(Reference Agrahar-Murugkar46) to 90%(Reference Durrani, Ahmad and Abbas47), and prevalence of thinness varied from 1·3%(30) to 63%(Reference Bharthi, Ghritlahre, Das and Bose48). Some publications determined prevalence of stunting and/or thinness with different growth references(Reference Agrahar-Murugkar46,Reference Garg, Kaur and Gupta49–Reference Sikdar53) . On the one hand, the prevalence of nutritional status varied greatly with the growth reference employed. Agrahar-Murugkar (2005) showed that stunting prevalence in Indian schoolgirls was 46% using Waterlow’s classification and 7% using ≤2 SD from median of CDC 2000(Reference Agrahar-Murugkar46). On the other hand, variability of thinness was found to be smaller by Garg et al. (2013), ranging from 6·6% (using <5th percentile of IAP reference) to 17% (using ≤2 SD from median of CDC 2000) among adolescent boys in India(Reference Garg, Kaur and Gupta49). Four publications comparing different references reported that nutritional status prevalence measured by IAP reference was lower compared with prevalence measured by other growth references such as CDC, WHO and NCHS(Reference Garg, Kaur and Gupta49–Reference Prashant and Shaw52).
Sex, age, socio-economic, demographic, dietary and other associated factors were evaluated in relation to stunting and thinness among South Asian adolescents. Four publications reported that stunting in adolescent girls was more common than in adolescent boys(Reference Mondal and Sen54–Reference Vashist and Goel57), but the difference was statistically significant in only one publication(Reference Pal, Pari, Sinha and Dhara55). In contrast, prevalence of stunting was found to be significantly higher among boys than girls in Northeast India, resulting in 1·55 times greater risk of being stunted for boys as compared with girls(Reference Rengma, Bose and Mondal58). Prevalence of thinness was found to be higher among boys than girls in eight publications(Reference Mondal and Sen54,Reference Vashist and Goel57,Reference Bose and Bisai59–Reference Mondal64) reporting significance in five(Reference Mondal and Sen54,Reference Bose and Bisai59–Reference Das, Ray, Joardar and Dasgupta61,Reference Mondal64) . Overall prevalence of thinness was slightly higher among girls than boys in two publications from India, but there was no sex-specific significant difference(Reference Bharthi, Ghritlahre, Das and Bose48,Reference Mondal and Terangpi65) . Indian adolescents showed a significantly higher prevalence of stunting with increased age(Reference Pal, Pari, Sinha and Dhara55,Reference Rengma, Bose and Mondal58) . The results of these two publications also showed that the risk of stunting in late adolescence was approximately two(Reference Rengma, Bose and Mondal58) to four times(Reference Pal, Pari, Sinha and Dhara55) higher than in early adolescence. Prevalence of thinness was greater among early adolescents compared with older counterparts(Reference Pal, Pari, Sinha and Dhara55,Reference Bose and Bisai59,Reference Das, Ray, Joardar and Dasgupta61,Reference Ameer and Chandrasekhar66,Reference Radhika, Kumar, Krishna and Laxmaiah67) . However, age-specific trends in thinness prevalence were absent in six publications(Reference Bharthi, Ghritlahre, Das and Bose48,Reference Mondal and Sen54,Reference Mondal64,Reference Mondal and Terangpi65,Reference Das and Biswas68,Reference Debnath, Tigga, Mondal and Sen69) . While stunting was more common amongst adolescents residing in rural areas in India(Reference Rao, Balakrishna, Laxmaiah, Venkaiah and Brahmam56,Reference Vashist and Goel57,Reference Choudhary, Khichar and Dabi70,Reference Maiti, De, Bera, Ghosh and Paul71) , thinness was found to be more prevalent in both urban(Reference Choudhary, Khichar and Dabi70) and rural settings(Reference Vashist and Goel57). Low socio-economic status was significantly associated with stunting(Reference Pal, Pari, Sinha and Dhara55,Reference Rengma, Bose and Mondal58) and thinness(Reference Pal, Pari, Sinha and Dhara55,Reference Niranjala and Gunawardena72) among South Asian adolescents. Thinness was significantly higher in adolescent girls living in households with unimproved water sources(Reference Radhika, Kumar, Krishna and Laxmaiah67,Reference Niranjala and Gunawardena72) . Parents’ education(Reference Rengma, Bose and Mondal58,Reference Khan, Nicola and Khan63,Reference Das and Biswas68) , birth order and family size(Reference Venkaiah, Damayanti, Nayak and Vijayaraghavan29,Reference Pal, Pari, Sinha and Dhara55,Reference Debnath, Tigga, Mondal and Sen69) as well as dietary determinants such as energy intake(Reference Ameer and Chandrasekhar66,Reference Hettiarachchi, Wickremasinghe, Hilmers and Abrams73) , micronutrient intake(Reference Radhika, Kumar, Krishna and Laxmaiah67,Reference Hettiarachchi, Wickremasinghe, Hilmers and Abrams73) , vegetarianism(Reference Ameer and Chandrasekhar66), dietary diversity(Reference Niranjala and Gunawardena72) and food availability(Reference Niranjala and Gunawardena72) were found to be significantly contributing to adolescent undernutrition.
Anaemia (low haemoglobin concentration) ranged between 1%(Reference Allen and Rodrigo74) and 98·3%(Reference Shridevi, Madhavi, Chandra Sekhar and Deotale75). Prevalence of micronutrient deficiency varied (Supplementary material 1); vitamin A deficiency from 0·3%(Reference Jayatissa76) to 65·4%(Reference Kawade77), folic acid deficiency from 1·5%(Reference Gupta, Kapil, Ramakrishnan, Pandey and Yadav78) to 54·6%(Reference Hettiarachchi, Liyanage, Wickremasinghe, Hilmers and Abrahams79), vitamin B12 deficiency from 0·44%(Reference Thoradeniya, Wickremasinghe, Ramanayake and Atukorala80) to 68·3%(Reference Kapil81), vitamin D deficiency from 70%(Reference Kapil, Pandey and Sharma82) to 96·3%(Reference Kapil, Pandey and Goswami83), vitamin C deficiency from 2%(Reference Ahmed, Khan, Banu, Qazi and Akhtaruzzaman36) to 10·8%(Reference Kawade77) and zinc deficiency from 28·8%(Reference de Lanerolle-Dias, de Silva and Lanerolle84) to 72·4%(Reference Kawade77). Prevalence of iodine deficiency was 23·6%(Reference Pandav, Mallik and Anand85) and 38·4%(Reference Harun-Or-Rashid, Yoshida, Morita, Chowdhury and Sakamoto86), prevalence of vitamin B2 deficiency was 89%(Reference Ahmed, Khan, Banu, Qazi and Akhtaruzzaman36) and prevalence of iron deficiency was 55%(Reference Kapil81).
Prevalence of micronutrient deficiencies and associated factors was studied among South Asian adolescents. Association between the prevalence of vitamin A deficiency and sex was not significant in two publications(Reference Jayatissa76,Reference Agrawal and Agrawal87) ; however, another publication found that xerophthalmia as defined by Bitot spots and/or night blindness was significantly associated with adolescent boys(Reference Sinha, Kulkarni and Nangia88). While iron deficiency(Reference Allen and Rodrigo74) and vitamin D deficiency(Reference Kapil, Pandey and Sharma82,Reference Kapil, Pandey and Goswami83) were significantly higher among females, vitamin B12 deficiency was significantly more common among males(Reference Chakraborty and Mani89). On age-wise categorisation, two publications did not find a significant difference in prevalence of anaemia among Indian adolescent girls(Reference Laxmaiah and Balakrishna90,Reference Selvarani91) . The increase in age with the increase in the prevalence of vitamin A deficiency(Reference Agrawal and Agrawal87) and iron deficiency(Reference Allen and Rodrigo74) was found to be statistically significant. Vitamin B12 deficiency(Reference Chakraborty and Mani89) and anaemia(Reference Selvarani91) were significantly higher among rural adolescents compared with their urban counterparts. Ethnicity(Reference Allen and Rodrigo74), occupation of the father(Reference Gupta92) and education of the mother(Reference Lamba, Misra, Agrawal and Rana93) were associated with anaemia. When categorised on the basis of working status, working adolescent girls from Sri Lanka were at nearly twice the risk of having folic acid deficiency and twice the risk of having zinc deficiency when compared with non-working adolescents(Reference de Lanerolle-Dias, de Silva and Lanerolle84).
4. Discussion
There are limited reviews on adolescent undernutrition in South Asia(Reference Khan, Bano and Salam17–Reference Mak and Tan20). To the authors’ knowledge, this study is the first scoping review mapping the available evidence. The search strategy involved seven electronic databases and grey literature. From 6181 records matching the search, 128 met the selection criteria and were therefore included in this scoping review.
The first aim of the present scoping review was to map the evidence on all forms of adolescent undernutrition in South Asia. Several characteristics of the included publications were examined. The evidence was considerably recent as there were only 12 publications from before 2004. There was available literature on adolescent micronutrient deficiencies since the 1970s; however, the oldest evidence calculating BMI-for-age and height-for-age was published after WHO guidance (1995) on measuring and classifying thinness and stunting in adolescents. Evidence included in this scoping review comprises exclusively quantitative methods employing mostly cross-sectional study design. Literature was clustered in certain states or provinces of some South Asian countries; most publications were conducted in India. Rural areas were the most reported communities. These findings show an uneven geographical distribution of the literature across countries and within countries in South Asia. Half of the publications were school-based, while 35% were community-based. Despite the large numbers of out-of-school adolescents in South Asia(94), recruiting participants from madrassahs, schools and high schools was possibly more accessible than a household survey or door-to-door approach. In this scoping review, we identified that undernutrition was more studied among adolescent girls than boys, and thinness was more studied than stunting or micronutrient deficiencies. In addition, anaemia was the most evaluated micronutrient deficiency.
This scoping review found only 12 publications that included an intervention component, one of which was a school feeding program and the rest were supplementation programs. The age limitation in this scoping review might have excluded some interventions targeting children from birth to adolescence. It is worth noting that we did not assess the effectiveness of these programs in reducing adolescent undernutrition as this falls outside the aims of our scoping review. However, all publications were successful in improving at least one form of adolescent undernutrition. Recommendations from these publications can be inferred. The authors of the school feeding program(Reference Thomas, Srinivasan and Sudarshan37) suggested that noting the exact length of attendance time to the program and quantifying meal intake by each student could be key to examining more accurately the nutritional benefits of school meals and developing detailed nutritional school programs. Recommendations resulting from the supplementation programs highlighted the need to explore long-term efficacy, dose and intake frequency of supplements to enhance adolescent micronutrient status.
A considerable number of publications that did not include an intervention component were available. These publications assessed the prevalence of all forms of adolescent undernutrition in South Asia. Undernutrition prevalence varied greatly. This may be due to the usage of different growth references, classifications and cut-offs as well as differences in characteristics of the target population such as age range and setting. Hence, comparison across publications cannot be made. It is important to note that this scoping review did not assess the quality of undernutrition indicators measured in the included publications; consequently, prevalence could have been overestimated or underestimated. Six publications nevertheless assessed and compared prevalence of stunting and/or thinness using different growth references(Reference Agrahar-Murugkar46,Reference Garg, Kaur and Gupta49–Reference Sikdar53) . The review findings showed that prevalence depended substantially on the growth reference used. The lowest thinness and stunting rates were given by IAP reference(Reference Garg, Kaur and Gupta49–Reference Prashant and Shaw52). Applicability of different growth references to Indian schoolchildren was recently examined(Reference Singh, Gandhi and Malhotra95). Similarly to our findings, Singh et al. (2020) observed that IAP reference classified fewer children as stunted and thin than WHO reference 2007. The authors suggested using IAP reference over WHO reference 2007 to evaluate growth in Indian schoolchildren. Further research on applicability of different growth references to South Asian adolescents should be conducted, to enable undernutrition prevalence to be compared across South Asian countries and worldwide.
Undernutrition-associated factors were evaluated in 38 of the included publications in this scoping review. The findings were presented with raw prevalence, P values and odds ratios, impeding comparison across publications. In addition, the findings were inconsistent for some of the associated factors. For instance, stunting was found to be more prevalent among girls, but evidence also suggested boys were at greater risk of being stunted. The findings on thinness were slightly more dependable, with boys being thinner than girls. Sex association varied greatly with the micronutrient deficiency studied. Stunting, thinness and micronutrient deficiencies were also associated with age, rural and urban areas, socio-economic status, sanitation, parents’ education and occupation, birth order, individual working status, family size and dietary determinants.
The second aim of this scoping review was to identify gaps in knowledge. We found that much of the available literature covers prevalence of adolescent undernutrition in the South Asian context using a cross-sectional design. This indicates that additional research on undernutrition using other study designs is needed to explore causality during adolescence instead of association. In addition, this scoping review did not locate any qualitative or mixed-methods publications. These approaches could contribute to better understanding adolescent undernutrition as well as evaluating if experiences from adolescents match with undernutrition-associated factors given by quantitative research. This scoping review also identified that there is a lack of adolescent undernutrition literature in Afghanistan, Bangladesh, Bhutan, Maldives, Nepal, Pakistan and Sri Lanka, as nearly 70% of the evidence included was conducted in India. Moreover, we observed that some publications were less systematic than others in reporting method and target characteristics such as age of the sample, community, setting or growth reference. As a consequence, this information was difficult to extract, and so it is missing. Reporting the community (rural, slum, urban or nationally representative sample) is a clear example, as we could not identify it in 40% of the publications. We noticed greater evidence scarcity from slums and national representative samples as well as research targeting out-of-school adolescents. This review suggests that supplementation and school feeding program research on South Asian adolescents seldom evaluated cost-effectiveness and impact on undernutrition rates in the long term on a large scale. Therefore, future research should aim to be implemented at a national level, also incorporating other types of interventions such as nutrition promotion, weight gain treatment, regulatory interventions (i.e. marketing) or health-related actions (i.e. deworming) to tackle adolescent undernutrition. This scoping review found that thinness (BMI-for-age) was more widely used to measure adolescent nutritional status than stunting (height-for-age). However, both indicators should be calculated since they assess different forms of undernutrition. Finally, existing literature did not appropriately determine whether some undernutrition-associated factors such as sex or age are risk or protective determinants. In addition, little is known about other factors such as dietary intake or sanitation in relation to adolescent undernutrition in South Asia.
Our scoping review has some limitations. Although our search strategy and selection process followed systematic methods, the challenges of searching for grey literature might have obstructed the inclusion of some relevant unpublished evidence(96). Due to time constraints and the large number of included publications, we did not search additional resources by hand searching the evidence reference list. Despite these limitations, we are confident that this scoping review provides a wide perspective on adolescent undernutrition in South Asia.
5. Conclusion
This scoping review located a broad range of publications on adolescent undernutrition in South Asia and identified noteworthy gaps in the evidence. Further exploration of additional methods to evaluate adolescent undernutrition in South Asia and its associated factors is necessary to implement effective actions for improving adolescent nutritional status.
Financial support
This work was supported by NIHR grant number 16/136/87. The research was commissioned by the National Institute of Health Research using Official Development Assistance (ODA) funding. The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR or the Department of Health and Social Care.
Conflict of interest
None.
Authorship
S.E.Q. conceived the idea and developed the methods. P.G., R.I. and L.A.K. contributed substantially to the conception and design. S.E.Q. conducted the searches, retrieved articles and screened evidence. M.K. and N.O. screened evidence. S.E.Q. conducted the analysis and interpretation of data. S.E.Q. wrote the first draft of the manuscript, and P.G. and L.A.K. supported the drafting and editing of the manuscript. S.E.Q., P.G., M.K. and N..O. revised the final manuscript. All authors approved the final manuscript.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1017/S0954422421000068







